October 2021
Welcome to the October issue of InFocus, where we provide insights and solutions to help sites and other stakeholders ensure site sustainability.
SCRS Current

Whew! The SCRS team is still beaming from an extraordinary Global Site Solutions Summit in Hollywood, Florida. Nearly 800 sites, CROs, solution providers, sponsors, and industry stakeholders joined us to discuss changes, challenges, and innovations affecting the clinical research industry right now. Thank you to everyone who helped us make this happen including our amazing members, attendees, exhibitors, and event sponsors. It was truly wonderful to be with so many of you again to celebrate the courage and resilience you have shown the world over the past two years. Congratulations again to the 2021 SCRS award recipients, and we look forward to sharing more information about each winner in our upcoming publications.
At the Global Site Solutions Summit, we also announced SCRS’ new Honorary President, David Vulcano. Vulcano brings nearly 30 years of experience within the clinical research industry and has been a member of the SCRS Leadership Council since 2015, providing guidance and direction for the company’s initiatives as a strong advocate for clinical research sites.
Next, we are excited to host the Oncology Summit in-person in Austin, Texas next January. Join us for insightful and data-driven content, thought-provoking breakout sessions, and engaging networking opportunities that proactively address increasingly complex challenges within oncology clinical research.
Also in this issue, listen to the latest SCRS Talks podcast episode featuring Stacey Bledsoe from Lilly as we discuss the important initiatives surrounding diversity in oncology studies. This month’s Site Buzz, covering popular discussions in the SCRS online Member Community, touches on the ambiguity and concern surrounding non-compete agreements in site contracts. Hear from NAVREF on how the organization established more efficient and collaborative practices throughout its operations despite the challenges of COVID-19. We will also share insights from Amgen and Merck on ways sites can modernize statistical analytics to drive innovations for patients.
As discussed in the Metrics that Matter article in this newsletter, we are seeing improvements between site, sponsor and CRO relationships, and SCRS hopes to be a catalyst for even more dialogue and better industry partnerships through our many programs and events.
Sites NOW, which was created at the 2020 SCRS Global Site Solutions Summit, has a full calendar that will continue through 2022. See the recap of our August presentation in this newsletter and be sure to attend the next meeting in November to be part of the discussion.
Metrics that Matter
Site, Sponsor and CRO Relationships
Each year, SCRS conducts the Site Landscape survey to gather data on the state of global clinical research sites related to key areas such as site partnership, communication, study start-up, feasibility, financials and technology use/adoption. The data are presented at the Global Site Solutions Summit and each annual Summit thereafter.
One of the key topics of the survey is relationships and communication between sites, sponsors, and CROs. When sites were asked how they feel they are treated today, 54% said they were treated “somewhat positive” by sponsors and 42% by CROs. However, 17% of sites said CROs treat them somewhat negatively, compared to only 7% of sponsors.
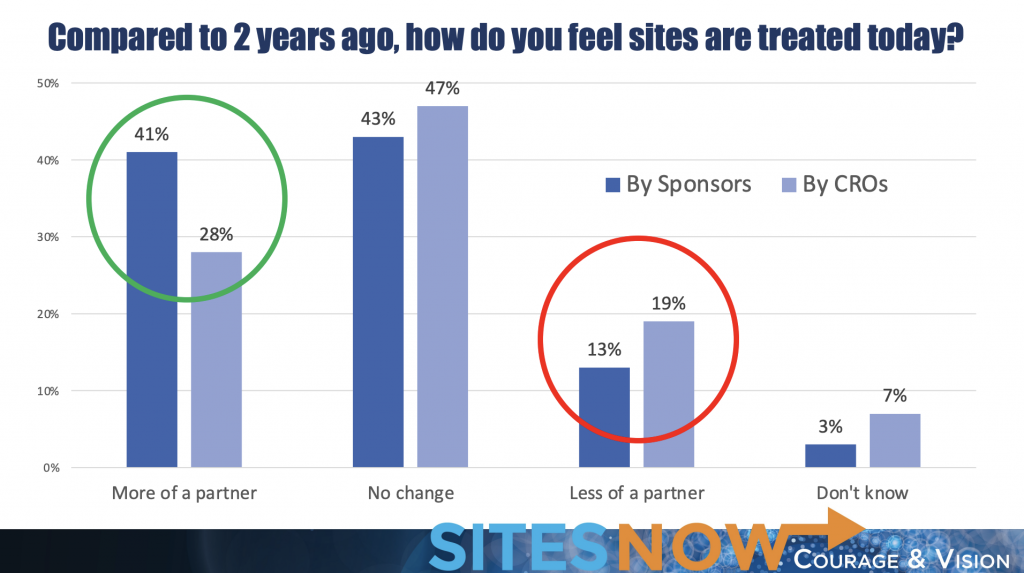
When reflecting on CRO and sponsor relationships two years ago, 41% of sites said sponsors now treat them as more of a partner, which is a notable increase. 28% of sites said CROs treat them as more of a partner compared to two years ago but 19% of sites said CROs treat them like less of a partner compared to two years ago. This variance may mean that some sites have negative experiences with some CROs, while others may be positive. It could also indicate that some CROs have improved their relationships with sites, while others may have fallen behind on maintaining positive experiences.
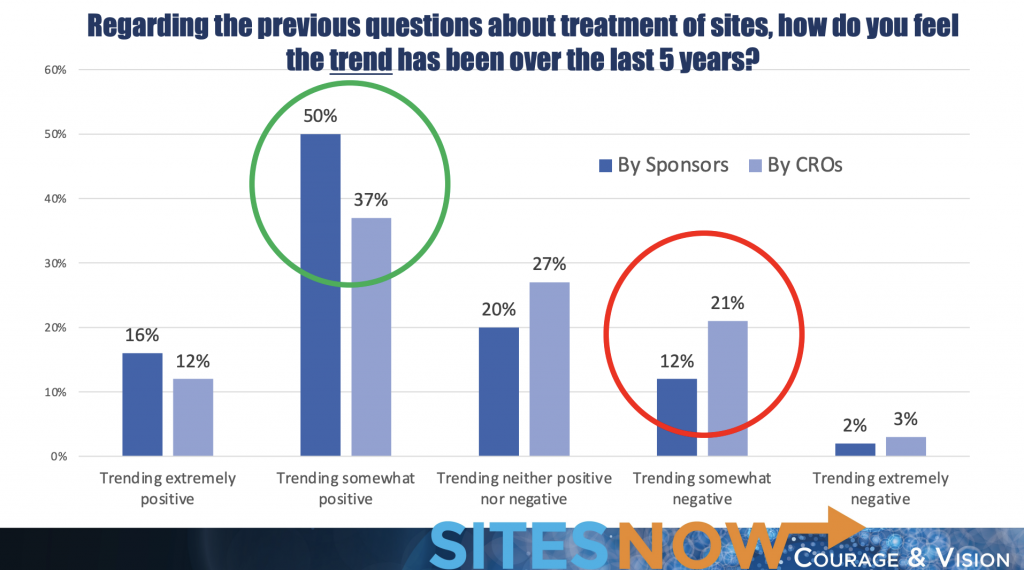
SCRS also asked sites to consider their relationships with CROs and sponsors over the last five years, and similar results were captured. It’s clear from the data that the relationship between sponsors and sites is trending in the right direction, but there is strong room for improvement for site and CRO relationships.
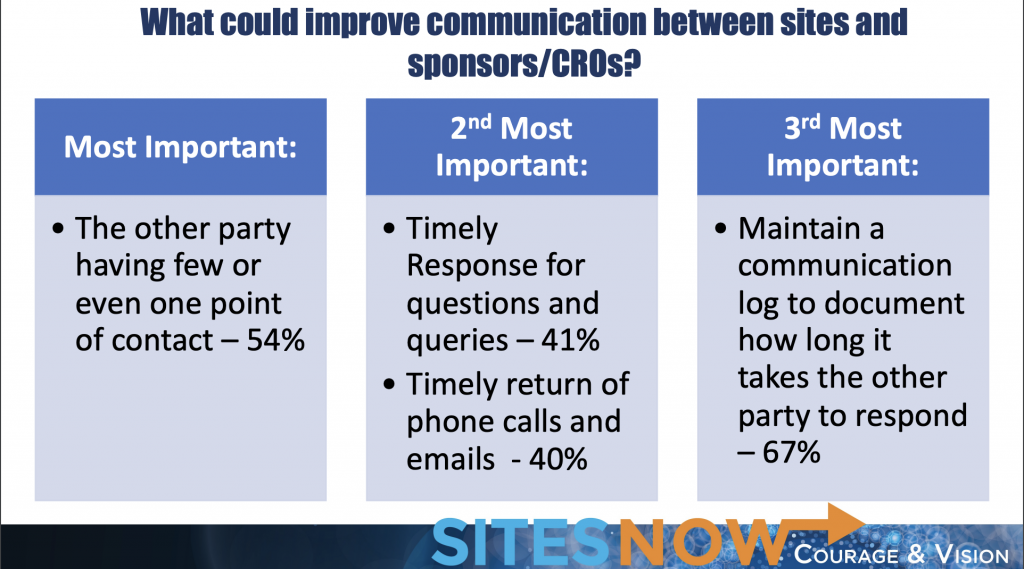
When asked what could improve the relationship between sites, sponsors and CROs, the majority of sites indicated that it was most important to them to have few or one point of contact with the sponsor or CRO. The second most important factor included timely responses to questions, phone calls and emails. Finally, maintaining a communication log to document how long it takes for the other party to respond was the third most important factor for sites in improving relationships with their sponsors and CROs.
These ideas to improve site relationships and communication between stakeholders are just some of the many solutions discussed at the Global Site Solutions Summit. As always, SCRS encourages sites to speak up and proactively communicate needs, challenges, and ideas to improve processes while working with sponsors and CROs in order to form long-lasting and mutually beneficial relationships. For more informative discussions, join us at each Site Solutions Summit and monthly Sites NOW meetings to discover innovative suggestions for sponsors and CROs to improve collaboration with sites.
Member Spotlight
SCRS Member Benefit Update
While the SCRS team and community prepare to gather for the upcoming SCRS Oncology Summit taking place January 2022, SCRS is pleased to announce that our member benefit, Response Evaluation Criteria in Solid Tumors (RECIST) is now available in Spanish, Mandarin and English!
These oncology training programs were created for staff conducting clinical research by the Clinical Imaging Team at Merck Research Laboratories to support understanding of response evaluation criteria in solid tumors (RECIST1) and response evaluation criteria in clinical trials with immunotherapies (iRECIST2). We encourage you to take advantage of this valuable benefit in the language of your choice!
This is a great time to be sure that your site is accessing the resources that are geared specifically to clinical research in the area of oncology. These member benefits include the RECIST modules, our many publications and your participation in our on-line oncology community. It is the perfect place to collaborate and support one another as peers, while working together with SCRS in our steadfast vision to cure cancer. Sharing knowledge, posing questions, and working through challenges are absolutely necessary when seeking solutions that will increase the momentum of oncology research.
We hope you and your team will capitalize on the investment of your SCRS Membership through the RECIST training modules, the online Oncology Community and our upcoming Oncology Site Solutions Summit by taking these easy steps:
- Visit myscrs.org to access the RECIST TRAINING MODULES
- Sign up to be part of the Oncology Community by sending a request to onlinecommunity@myscrs.org
- Register for the Global Oncology Site Solutions Summit taking place in Austin, Texas January 28-29, 2022
SCRS truly hopes you will take full advantage of the benefits we offer with your membership. If you have any questions or need any assistance, we are here to help! Reach out to our Member Engagement Specialist, Anne Marie Molster at annemarie.molster@myscrs.org or call 410.696.5080.
SCRS Talks
A Chat with Michele Cameron, 2021 Christine K. Pierre Site Impact Award Winner
The Christine K. Pierre Site Impact Award recognizes an industry leader who has made tremendous contributions to the site community through their dedication, actions and innovation. They exemplify the mission of SCRS to unify the voice of the global clinical research site community for greater site sustainability and uphold the pillars of SCRS by supporting the education, advocacy, mentorship and connection of clinical research sites. Michele Cameron, BSN, MBA, CCRC, Director of Clinical Research at Clearwater Cardiovascular Consultants, embodies the essence of this award. We sit down with Michele, this year’s CKP Site Impact Award recipient, to learn about her site, how she has grown that organization to what it is today, and how winning this award has impacted her and her site.
Listen to the full SCRS Talks episode below.
Diversity in Oncology – Changes That Impact the Industry & What Must Be Done
Stacey Bledsoe, Advisor for Clinical Trial Diversity at Eli Lilly and Company (Lilly), joins us to talk about what is being done in the industry to advance diversity in clinical trials. Stacey touches specifically on some of the work Lilly is doing, as well as diving specifically into the Oncology therapeutic area. The needs of this community are different, yet just as important as others yet at times does not get the attention it needs. Stacey gives us lessons that we can apply to our trials at both the Sponsor and CRO level, as well as the site community.
Listen to the full SCRS Talks episode below.
SCRS Sites NOW
Sites NOW Recap: The Impact of Successful Site Relationships
August’s Sites NOW topic, “The Impact of Successful Site Relationships”, focused on the critical importance of site partnerships and shared ideas on how sponsors, CROs, and solution providers can better work with sites to ensure efficient and effective communication and collaboration.
A common theme throughout the discussion reiterated that relationships with sites should aim to always be helpful and collaborative with trust and respect as key foundations. For a relationship to be successful, sites need to be heard and respected.
Where sites want to be heard and share their feedback depends; some want to be involved in protocol design, others want to contribute to operations. Ultimately, it’s about bringing in site input at the right time. However, sponsors and CROs should always consider what sites are sharing as leading indicators and activities that will not work for the protocol. By bringing sites into the development stage, sites can recognize potential barriers to recruitment and retention and can offer solutions. If CROs are unable to build in a site’s request, explain why so sites can better understand why that solution may not be feasible currently.
Sites have continued to share feedback that they would like CROs and sponsors to interact with them as partners as well as focusing on better attention to detail, understanding timing of cycles, and “owning” the experience of working together. One way to ensure this would be for sponsors and CROs to have dedicated employees to facilitate the experience between the investigator and site. Every site needs to know who to contact – who is the right person in various aspects of the business that sites can reach out to?
Sites also mentioned that minimizing new training needed and working with sites on their existing systems benefits all stakeholders in clinical research. Sponsors and CROs can trust sites’ experience and knowledge and shouldn’t require training for every new tool. Panelists encouraged sites and CROs to question what’s in the protocol to determine if a process, form, widget, etc is still needed, especially when it doesn’t add value and burdens the site. When implementing new technology at the site, consider three questions: “How does it make site data better, workdays a little easier, and our patients better off?”
Another common theme in this discussion advocated for sites to proactively share their expectations, challenges, and needs. This can happen in the contract negotiation stage, throughout the study, or after completion to help sponsors and CROs better understand where bottlenecks may exist, as well as ideas to improve future trials and ultimately their relationship with sites.
Part of the SCRS mission is to advocate for better site relationships by being an active partner in industry-wide initiatives and dialogues. Join us in our mission by engaging in the important discussions at Sites NOW meetings, convening each month. Learn more at https://myscrs.org/scrs-sites-now/
Sites NOW August panelists included Darren Cowan, Area Head, Americas Global Site & Study Operations at Pfizer, Karen McIntyre, Global Lead Catalyst Program and Site Relationships at Syneos Health, and David Vulcano, Vice President, Research Compliance & Integrity at HCA Healthcare.
SCRS Connects
SCRS chatted with Rick Starrs, CEO of the National Association of Veterans’ Research and Education Foundation (NAVREF), to discuss how the organization has pivoted and prevailed throughout its 30-year history.
Founded in 1992 to support Veterans Affairs (VA) affiliated nonprofit corporations (NPCs), the National Association of Veterans’ Research and Education Foundation (NAVREF)’s mission is to support veteran health through education, communication, and advocacy. The organization has spent the last 30 years helping VA-affiliated NPCs share best practices to establish themselves and grow while advocating on their behalf with Congressional and federal agency leaders.
NAVREF’s members are affiliated with VA medical centers by federal statute. They support the nation’s largest integrated healthcare system consisting of nine million beneficiaries and 100+ active research sites nationwide. NAVREF serves as a matchmaker, connecting companies with interested VA-affiliated NPCs, investigators, and study sites. NAVREF also bridges the gap between VA and the biomedical industry by helping VA understand the needs of pharmaceutical and biomedical firms and by helping those firms more easily navigate VA.
There are currently 79 VA-affiliated nonprofits across the country and they are most often located on the VA campus and inside VA research buildings where they work hand-in-hand with VA research services. As a group, the NPCs handle approximately $285 million/year in support of veterans’ research and education activities.
In 2017, VA Chief Research and Development Officer Dr. Rachel Ramoni made enhanced access to clinical trials for veterans one of her organization’s top priorities. NAVREF and the Office of Research and Development worked in tandem to initiate the Access to Clinical Trials for Veterans (ACT for Veterans) initiative in 2018. This initiative brought together VA investigators, NPC executives and VA research leaders with pharmaceutical companies, CROs and patient advocacy groups to explore how to establish an enterprise-wide approach to external partnerships and facilitate policies and operational changes for multi-site clinical trial start-up.
The first multi-site clinical trial on US soil was led by VA in the 1940s. Since that time, VA has operated a robust and productive cooperative studies program. However, these research activities were primarily funded by VA and focused internally. Engagement with industry-funded clinical research was inconsistent and varied from one VA hospital to the next. Pharmaceutical sponsors were challenged by uneven information flow, unpredictable timelines, and local variability.
To address these challenges, the ACT for Veterans initiative brought in industry stakeholders to get feedback on how VA could be a better partner for industry-sponsored studies and how to become a partner of choice. Based on the industry feedback received, VA started addressing gaps and building processes with a focus on externally sponsored clinical trials. After identifying 13 areas that needed improvement, they formed five work groups that included industry partners, VA researchers, and nonprofit executives and started on those work streams.
Work group accomplishments included gaining VA approval for a new policy permitting use of commercial IRBs, implementing a new streamlined process for centralized execution of Confidential Disclosure Agreements, and establishing a new office—the Partnered Research Program—focused on facilitating externally sponsored research with industry partners.
“We were very excited about that, but it happened shortly after COVID entered the country, so the new Partnered Research Program focused entirely on COVID activities,” Starrs said. In March 2020, the VA stopped all non-essential research. Clinical research that could not be transitioned to virtual studies was put on pause and there were no new starts for clinical research, which accounts for most of the externally-sponsored research at many VA sites.
“We were fearful about what the financials for fiscal year 2020 would look like since there was a significant decrease in the amount of research that our organizations could support,” said Starrs.

Fortunately, many sites were able to transition to externally funded COVID research. Across the VA, there were over 300 research projects across 70 VA sites. In just the first seven months of 2021, VA authors published 316 scientific papers based on COVID research activity.
Pre-COVID, one of the ACT for Veterans initiative’s primary goals was to get 100 days faster in VA study startup timelines. “VA used a lot of the processes the work groups put in place, such as the use of commercial IRBs for these COVID studies, and they were able to start up COVID trials in 30 days or less which was a remarkable improvement from where they had been pre-COVID,” Starrs shared.
“VA may not necessarily be able maintain a 30-day startup, but they’re not going to go back to the days of 250-day average startup timelines. We are confident VA can stay 100-days faster and really be competitive.”
Starrs said he recently received feedback from a CRO they work with who commented that over the last year, study timelines at VA sites have improved by more than 100 days and were comparable to or better than academic medical center timelines.
During the first two years of the ACT for Veterans Initiative, NAVREF recognized that communication between VA and the biomedical industry community needed to improve. This year, NAVREF rolled out the Industry Partner Consortium to provide a regular and recurring forum for VA research leaders, nonprofit executives and industry partners to exchange information, share priorities, build relationships and offer insight into challenges and opportunities.
“The establishment of the Partnered Research Program as the single, centralized point of entry to VA research has been very helpful. The ability to use commercial IRBs in certain circumstances has led to reduced timelines, so that’s been very positive. Now with the rollout of the Industry Partner Consortium, we look forward to improved communication and information sharing to help make the whole process smoother and more collaborative,” Starrs said.
Learn more about NAVREF and the Industry Partner Consortium.
Site Buzz
Non-Compete Agreements

With the rise of new and competing technologies in development, non-compete clauses have been popping up in site contracts from CROs and sponsors. This has created some hesitation and confusion among research sites, who shared input and experience with these non-compete agreements in the SCRS member site community.
An example of a non-compete clause sites may encounter: While this AGREEMENT is in force, the INVESTIGATOR, any designee, agent and/or the INSTITUTION agree(s) not to participate in a clinical trial for a competitive device.
However, the ambiguity with these clauses can be too risky for sites as there may be confusion on what is the definition of a competitive trial. If the CRO considers competitive trials those that have exactly same or overlapping eligibility criteria, potential subjects that can be enrolled in two or more studies in a site’s study portfolio may technically mean a site has competitive trials. Any device could be deemed competing if it treats the same indication or condition as the language is very vague.
In other industries, non-competes typically come with additional benefits for both parties. For instance, a non-compete clause might be attractive to a site if they will be paid a premium price and/or a non-refundable retainer fee to compensate for any potential lost revenue. Additionally, non-competes may give sites additional leverage to say the sponsor will not use other sites within your greater recruitment catchment area of your location without giving you the right of first refusal to do the study, for example. Sites will avoid signing any loose contracts that are not in their best interest – so CROs and sponsors should avoid non-competes unless additional financial compensation is offered and definitions of “competing trials” are iron-clad.
Modernizing Statistical Analytics
7 Ways to Modernize Statistical Analytics to Drive Innovations for Patients
By Daniel Woodie, Merck; and Min Lee, Amgen
The increasing number of data sources collected as part of drug development and commercialization has vastly increased the amount and types of data collected. This has increased the need and urgency for technology that can process large amounts of structured and unstructured data to support regulatory filings.
Many companies have existing standard operating procedures for common off-the-shelf commercial offerings. However, the increasing complexity of data analysis that is now required often means that companies need to modernize their statistical offerings through nontraditional (e.g., open-source) software.
As such, TransCelerate BioPharma recently produced a Modernization of Statistical Analysis (MSA) Framework that can be used as a methodology that demonstrates to health authorities that these nontraditional software capabilities are reliable. Our goal is to build confidence in cutting-edge analytical programs, which will ultimately help bring new medical breakthrough products to patients more quickly.
This framework has been developed to provide practical guidance on how to demonstrate that an analytics solution produces credible results and is suitable for use in the regulatory process. TransCelerate has garnered a few key takeaways for sponsors to consider when looking to introduce non traditional statistical analytical tools to their clinical trial processes.
Below are seven tips that can be used to help implement a modernized statistical analysis environment:
- Focus on Accuracy, Reproducibility, and Traceability
Prioritizing these three core principles of the MSA framework helps ensure that the system is able to support regulatory decision-making and generates reliable results. For our purposes, accuracy is defined as the measure of correctness of software libraries that are used to generate results from an MSA environment, not the programs that developers write using those software libraries. Reproducibility then is demonstrated by showing a statistical analysis output that can be recreated from the original data set along with the associated environment, including all artifacts and dependencies. Traceability refers to the ability to trace inputs to outputs, with the main goal being to provide evidence that connects data, code, and environment to the final output that is produced.
It’s important to note that the three principles do not work in isolation — rather, they must be fully integrated together to achieve success. Ensuring this full, end-to-end control will provide health authorities with confidence that the MSA system is reliable.
- Build on Legacy Systems
While the concepts in the MSA framework can be basic in theory, they may prove challenging to implement as current environments in biopharmaceutical companies often use existing legacy technology, which can perpetuate a mindset to reinforce the status quo and thus can act as a check on the evolution of analytical programming languages.
For companies looking to modernize their legacy systems, creating a set of best practices and exploring potential use cases before implementation can be invaluable. Preparing materials such as these can also help develop support among senior leadership, which will allow for the modernized systems to be introduced easier and seamlessly.
- Create a Hand-Off Between Business and IT
IT traditionally builds and maintains computing environments based upon SOPs and other defined processes that ensure reliability, which might be different than the tools or automated processes followed on the business side. As such, there are risk and change management aspects to consider since these two sides of the coin may not be following the same process for the statistical analysis environment.
Collaboration across the organization can expedite timelines and improve the implementation of new technologies that have actual impact on business outcomes. A crucial factor to ensure a smooth transition and hand-off between business and IT is for the senior leadership of both units to create a partnership early on and define clear roles and responsibilities for the cross-functional team members.
- Create and Document Any Efforts as Part of the MSA
Creating a culture that automatically collects, collates, and shares documentation on how analytical processes are implemented is crucial. This will help demonstrate to stakeholders in this environment, including health authorities, that the lineage of dependencies relied upon by statistical analysis systems are valid. Documentation guidelines, best practices, and processes need to be clearly defined and required as part of the MSA analysis from the beginning of the programming life cycle. Programmers should be documenting as they develop, test, and report.
- Create Clear Roles Within an MSA Environment
There are various roles involved in creating an MSA and moving it forward. From statisticians to engineers and clinical quality experts, modernization of statistical analysis requires many roles to work together to advance and improve the statistical computing environment.
Open communication among business functions is imperative to identifying and assigning these roles, and delivering the MSA framework depends heavily on collaboration among working teams. As mentioned above, one of the critical key success factors is ensuring that roles and responsibilities are clearly defined. Communication and collaboration ensure effective coordination between stakeholders, and, ultimately, help deliver an effective MSA environment.
- Develop a Testing Driven Programming Strategy
The level of testing required for the MSA environment is critical to ensure its success. There is a balance between too much testing – that leads to an inflexible environment and can hinder a programmer’s ability to leverage the packages they need for deliverables – to too little testing that may compromise confidence in the software’s reliability. A thorough examination of where risk is high versus low and experimentation will be key to strike the right balance. A key component of obtaining the right balance is to have cross-functional alignment and input from multiple teams such as quality, compliance, biostatisticians, programmers, IT, etc.
- Focus on Culture
None of this is possible without embracing a mindset for innovation. Support for modernization must come from all levels of the organization, including senior leadership. This is crucial to conduct the necessary changes in order to remain on the cutting edge of statistical analysis.
Collectively, these seven tips — outlined further in our framework document — can help companies implement these technological innovations that ultimately improve the ability to bring lifesaving medicines to patients more quickly.
While each organization will have to determine its own desire for change and willingness to balance any risks, embracing a culture shift toward modernizing statistical analysis will be crucial to the ability to bring medicines to patients more quickly.
About SCRS
Founded in 2012, SCRS is a global trade organization that unifies the voice of the clinical research site community to create greater site sustainability. Representing over 9,500 sites in 47 countries, SCRS membership provides sites with a community dedicated to advocacy, education, connectivity and mentorship. SCRS is an influential voice for sites and an active partner in industry-wide initiatives and dialogues focused on improving the clinical research enterprise. Our Voice. Our Community. Your Success. Join the community.
