July 2021
Welcome to the July issue of InFocus, where we provide insights and solutions to help sites and other stakeholders ensure site sustainability.
SCRS Current
The SCRS team is still beaming from our two-day virtual Asia-Pacific Site Solutions Summit. This was our third virtual summit and once again, we were pleased to see a record number of registrations – nearly 800 clinical research industry stakeholders across 25 countries! Now, the SCRS team is busy planning for the 2021 Global Site Solutions Summit in Hollywood, Florida, which will be our first in-person event in over a year. Since it will be the first time we will be able to get together in over a year, we’re planning to make this year’s Global Site Solutions Summit the best ever! We’re kicking things off at this year’s Global Summit with sites-only symposiums on September 30, while the following three days will include inspirational keynote session, workshops, breakouts, networking, and prestigious awards ceremonies. Register today to make sure you don’t miss out!
To help us make the 2021 Global Site Solutions Summit our best yet, we encourage you to share your experience in the 2021 Site Landscape Survey, which is open to everyone in the clinical research industry. We also are looking to hear site’s voices in the new Decentralized Trials Site Survey which was developed as part of the SCRS Digital Innovation Initiative. Results and insights from both surveys will be presented at this year’s Global Summit and will inform future SCRS advocacy, publications, and so much more. Please take a moment to make your voice heard!
We have many additional initiatives in progress including the continuation of Sites NOW meetings, which have provided an engaging platform for sites and industry stakeholders to discuss and suggest solutions for important topics the industry is facing. The SCRS Digital Innovation Initiative is also moving forward quickly with its plans to produce informational documents for sites on how to effectively utilize decentralized trial technology and connected devices.
Please also join us in welcoming SCRS’ newest team member, Anne Marie Molster, as our Membership Engagement Specialist. Molster joins SCRS to increase membership value for our site community, which includes ensuring members are aware of the benefits available to their entire research team through SCRS. In addition, she will explore ways to expand SCRS’ offerings based upon the community’s expertise and ideas. If you would like to learn more about SCRS membership and the many benefits we offer, please reach out to members@myscrs.org.
Member Spotlight

This month, SCRS membership wants to be sure that you know about one of SCRS’ most important benefits available to you and the rest of your team: as a valued SCRS member, you are extended a significant discount upon registration for the Global Site Solutions Summit.
Attending the Global Summit not only offers you an opportunity to receive contact hours and continuing education credits, it is also a unique time for you to make significant connections while building relationships with sponsors, CROs, and solution providers.
This year’s Summit will be held in-person at the beautiful Diplomat Beach Resort in Hollywood, Florida. The Global Summit, being hosted October 1-3, will be filled with exciting keynotes and informative plenary sessions, workshops, breakout discussions, networking, prestigious awards ceremonies, and so much more. Sites can also attend Sites-Only Symposiums starting September 30.
If you do not have the SCRS site member discount code for registration, please reach out to members@myscrs.org.
If you have any questions about SCRS member benefits, our new Membership Engagement Specialist, Anne Marie Molster, will be happy to assist you. We are incredibly excited about how this year’s in-person Summit is developing into the best ever! We are extremely proud to offer content that cannot be found anywhere else. We look forward to seeing all of you there!
SCRS Connects
SCRS Connects with Dr. Daniel J. Fox on the Importance of IVDs for Clinical Research

Daniel J. Fox, MPH, PhD
Director, Clinical Research
Springfield Clinic
Sites are often offered laboratory-based trials, specifically In vitro diagnostics (IVDs). Typically, a sponsor performing IVD studies collects blood samples to develop detection diagnostics and help to improve healthcare. These are quick research protocols that generally comprise consenting a patient, drawing their blood or a tissue sample, and finally shipping specimen to the sponsor’s laboratory to develop their innovative diagnostic tests to help improve laboratory detection or identify diseases.
Many physicians, however, are hesitant to participate. Daniel J. Fox, MPH, Ph.D., Director of Clinical Research at Springfield Clinic shared with SCRS that he has frequently heard physicians respond to IVD study opportunities with disinterest, citing reasons like, “This has no direct benefit to our patients”, “This is just a blood draw study” or “It’s boring and it won’t pay well.”
Yet, many of these studies are cancer studies that focus on metastatic stage four and end-stage cancer patient populations. Recalling interactions Dr. Fox has had with patients participating in these types of studies, he said, “We’ve had patients with tears in their eyes saying they were so glad they had an opportunity to help the future of medicine. Some of them feel it may be one of their last contributions to this world.”
Often, the patients who meet these inclusion and exclusion criteria have severe cancer or an irrecoverable condition. When asked if they want to participate in a trial, many patients agree without question, conveying that they are grateful to help the future of medicine, and hoping they may prevent the same fate to future patients.
With that kind of impact, are denying these types of studies something that physicians should decide on? “I truly believe in patient-centric research, and not providing the patients with these opportunities is a problem,” said Dr. Fox. “We can’t just say these types of studies will not help patients. That’s not our right to say. It’s solely a decision the patient should have the right to make.”
The Benefits of IVD Trials
- Length: Trials tend to have a higher chance to generate margins if they are shorter. The shorter the trial, the more likely you will create a margin, primarily because there’s less of a chance of something costly happening before the study is completed. The shortest trials possible are blood draw studies. If negotiated properly, blood draw studies are an excellent series of studies to add to a site’s portfolio.
“You can’t get any shorter than a single consent and blood draw. There is some document keeping, but it is quick. Therefore, if you perform and negotiate them correctly, they can be highly competitive studies,” said Dr. Fox. - Portfolio Diversification: It’s critical to perform a variety of studies to balance clinical site activities. IVD studies are easy to fit within a flexible time window and may therefore become the foundation to a site’s financial stability. Sites can schedule these studies between “filler times” among other patients scheduled throughout the week. “It actually improves the efficiency of your staff and their utilization. Between the possible competitive margins that you can accumulate and the unwasted idle time between study visits, most sites will enjoy a lot of flexibility and resource efficiency as they perform these studies,” expanded Dr. Fox.
- Site and Staff Experience: One of the cornerstones of research now is laboratory diagnostics. Aside from outcome studies, a sample collection consent trial is one of the simplest to complete. These types of trials can give physicians the opportunities to grow professionally and develop the experience they need to perform more complex studies. IVD trials may also help clinical sites grow the expertise needed to expand their study offerings.
Dr. Fox shared his research site’s experience, saying, “We have had complex trial offers before from large pharmaceutical companies, such as heart failure studies or other complex clinical trials, and although we had interested physicians, we were not awarded the study. We weren’t awarded these complex studies because our interested physicians didn’t have the necessary clinical research experience to participate as PIs. IVD may help to grow physician investigator experience so they may be future PIs in complex studies. They are a critical cornerstone to helping physicians grow as investigators and clinical site portfolio grow in diversity.”
There are many other reasons to consider allowing these studies into a site, but it’s important to also address the negative stigma surrounding these types of studies.
Addressing Negative Stigmas for IVDs
“I think it’s really important to raise awareness into what we are not doing with IVD studies,” said Dr. Fox.
The industry is likely familiar with sample brokers, which Dr. Fox addressed. “There’s a huge market out there for sample brokers who sell remnant samples. These companies collect samples and then sell them to the highest bidder,” shared Dr. Fox. “I’ve seen the advertisements and articles, however there’s a huge stigma with this practice for clinical research sites, primarily because of the ethics behind the consent process during the commercialization of these samples. Because of the sample broker model, some sites may think the IVD study process is cheap, not worthwhile, and unethical. I think it’s really important to specify that IVD clinical trials are not sample brokerages. They are highly-regulated IRB-approved trials, they have a consent process, the patient is always in control of their samples, and samples are not commercialized, but instead used only for the purpose specified in the protocol and the patient’s consent form.”
How Sites Can Help Improve the Future of Clinical Research
Diagnostics lab tests are one of the founding pillars in research and they will only continue to get better if we support their advancement. “It is imperative to have this line of research progress with the rest of the field so that we can perform research better,” shared Dr. Fox. “In 10 years from now, what if someone invents a better CBC? How can we incorporate this new diagnostic technology into our trials if we don’t test it in its own clinical trial process?”
“It’s up to us to make IVDs, tailored medicine, and companion diagnostic studies better,” concluded Dr. Fox. “We’re trying to do research, we’re trying to help people, and we’re trying to advance medicine.”
Sites, we’d love your perspective on this. Are you offered many of these types of studies? If so, why do you or do you not participate? We’d love to hear your thoughts. Let us know in the SCRS Site Member community, or reach out to communications@myscrs.org.
SCRS Talks
Each year at the Global Site Solutions Summit, SCRS selects the Site Patient Recruitment Innovation Award (SPRIA) and Site Tank award winners. The Site Tank awards recognizes a site that has developed an innovative technology idea that enhances, empowers, or improves operations and transforms their site’s business. SPRIA acknowledges sites that have developed a new and original recruitment method or that have utilized a unique approach to recruitment success.
In this episode of SCRS Talks, we chatted with last year’s SPRIA and Site Tank award recipients, Clinical Trials of Texas and Accelerated Enrollment Solutions, as they reflect on what receiving these awards has done for their organizations, how they have tackled the challenges 2020 presented, and what they are doing to prepare for the future of clinical research.
Listen to the podcast now.
SPRIA and Site Tank submissions are open through July 31, 2021. Three finalists for each award will present at the 2021 Global Site Solutions Summit where the winner will be selected, announced and celebrated.
SCRS Sites NOW
Reimagining Study Performance Through Education
Revising Outdated Training Approaches Can Help Sites Achieve More:
Sites NOW June Session Tackles Reimagining Study Performance Through Education
 By Robert Geckeler, Product Director, ScienceMedia
By Robert Geckeler, Product Director, ScienceMedia
Delivering effective study training is particularly important in today’s clinical research climate, given the increasing complexity of study protocols and the trend toward utilizing more remote study elements adopted during the pandemic. This new environment provides the perfect opportunity for sponsors to begin reimagining training as a way to improve study performance.
At a high level, study training provides the context for an investigational trial by explaining the underlying rationale for the study. It details the study drug, its mechanism of action, and its intended use in the population of interest. It also helps clarify the objectives and goals of the study by linking specific study requirements and assessments to those objectives. Ensuring that these requirements, measures, and assessments are clear is the best way to facilitate site performance. But it is equally important to update sites with any necessary clarifications or on changes to those requirements when amendments occur, and that is why it is vital to be able to provide ongoing training as a study progresses.
Recommendations on training from the Clinical Trials Transformation Initiative (CTTI) suggested goals like moving away from a one-size-fits-all approach to account for different roles and varying levels of experience of study staff. Study training systems that allow role-based lesson assignments and incorporate assessments go a long way toward meeting this objective. Likewise, rather than generic check-box training, study-specific educational programming tailored to the protocol and the needs of the site can empower sites and truly facilitate the conduct of a quality clinical trial. While CTTI recommendations discuss advance evaluation of a site’s preparedness to conduct clinical research, that can reasonably be extended to mean applying some measure of comprehension and readiness for the trial based on study-specific knowledge. That means study training would ideally ensure, as part of the training process itself, that lessons had been effectively delivered and understood by all members of the study team.
Thorough and effective training primarily makes an impact by improving protocol compliance. Site staff who fully understand study requirements are more likely to adhere to them and avoid deviations and errors. Similarly, training works to support patient safety by ensuring that study staff and patients fully understand dosing schedules, expected risks, and the handling of adverse reactions. Improved compliance will lead to reduced variance, thereby improving data quality. Together, these improvements should also result in less time spent by sites on resolving data discrepancies and other inefficiencies.
The risks of inadequate training are exacerbated in an environment characterized by increasing protocol complexity and reliance on decentralized approaches, further emphasizing the need for improved training in today’s climate. For example, sites will vary in their prior experience using specific remote tools; adequate training can help level the field and reduce the burden on sites as they adopt these methods. Patient considerations like access to broadband Internet and technical ability come into play with the increasing use of remote approaches, and helping sites to account for and manage these in advance can save time and effort in the long run.
Many sites are bearing the burden of educating patients and implementing technology. Even though the technology aims to be more convenient to the patient, we are now asking patients to undertake activities that they would have experienced at the site. Study training that extends to providing instruction for patients lessons that effort for the site and may mean that sites are called upon less often to act as the ‘help desk’ when purely technical questions arise about the use of these tools.
However, many studies continue to rely on outdated training methods. After 30 years of having standard slide presentations serve as the primary tool for training, a change is clearly needed. Looking at CTTI’s recommendations, educational programming for a study should look beyond traditional methods and apply adult learning best practices. The use of cutting-edge training technologies that employ these principles helps guarantee that study education is as engaging, interactive, and effective as possible. CTTI guidelines also call for focusing study training so that the time and attention given to the most critical aspects of the study are weighted in proportion to their relevance. Modern training approaches that take a risk-based focus to protocol education deliver on this goal by zeroing in on those aspects of the study that have the greatest impact. This is further achieved through the use of modular training focused on discrete topics, or microlearning.
Platforms that offer flexible delivery allow site staff who are pressed for time to benefit from the convenience of on-demand access to study training. And as staff turnover occurs, new team members have access to the same level of training as those who were on board at the outset of the study. Along the same lines, one-and-done training at the commencement of a study is insufficient. Lesson reinforcement through repetition is critical to ensuring that peak levels of knowledge are maintained throughout a trial, and approaches like the microlearning format are ideally suited for that. Support tools like quick reference guides also serve as a way to reinforce study requirements.
By reimagining study training, sponsors and CROs have the opportunity to improve the support offered to sites, allowing them to better leverage site expertise by enhancing the efficiency and performance of study staff. A new approach to study education, which takes advantage of the latest standards for training content and delivery, has the potential to improve not only the site’s experience and patient satisfaction, but the quality of the study overall.
Join the next SCRS Sites NOW meeting, convening every month. For details, visit myscrs.org/scrs-sites-now/.
June 2021 Sites NOW Panel:
Philip Bedrin, Director of Medical & Clinical Learning Strategy & Solutions, ScienceMedia
Doug Schantz, Vice President, Clinical Operations, Alexion
Michele Cameron, Director of Clinical Research, Clearwater Cardiovascular Consultants
Sources: Bechtel, J., Chuck, T., Forrest, A., Hildebrand, C., Panhuis, J., Pattee, S. R., . . . Swezey, T. (2020). Improving the quality conduct and efficiency of clinical trials with training: Recommendations for preparedness and qualification of investigators and delegates. Contemporary Clinical Trials, 89, 105918. doi:10.1016/j.cct.2019.105918
Sites NOW Supporters













































Sites NOW Participating Organizations
AbbVie | Accel | Accellacare (ICON Plc) | Advarra | AstraZeneca | Bio-Optronics | BRANY | Bristol Myers Squibb | BTC/ClinEdge Site Networks | Clearwater Cardiovascular Consultants | ClinEdge | Clinical Site Partners, LLC | Clinical Trials of Texas, Inc.(CTT) | Clinical.ly | Clinvest Research | Complion | Covance | CTMD Clinical Research | DM Clinical Research | East Coast Institute for Research | Elite Research Network | Evolution Research Group | GlaxoSmithKline | Greenphire | HCA Healthcare | ICON, plc | Janssen R&D | LMC Manna | Research Medical deScriptions | Medix | Medpace | Meridian Clinical Research | Northwell Health | OrthoIllinois | Oviedo Medical | Research | Parexel | Pfizer | Pharmaseek | PMG Research | Prime Site Research Solutions | RealTime Software Solutions | Ripple | Science Corporation | Roche | RX Trials | Sanofi | Sentral Clinical Research Services | South Broward Research | StudyKIK | SubjectWell | Suncoast Clinical Research | Syneos Health | Synergy Clinical Research | Total Clinical Trial Management | Trifecta Clinical | Veeva | Veterans Research Foundation of Pittsburgh | VirTrial | WCG | PharmaSeek
Metrics That Matter
Improving Access to Research for Diverse and Underserved Populations
This is an excerpt of a whitepaper published by Advarra. To see the full paper in its entirety, please download it on the Advarra website.
Increasing diversity and inclusion in research has long been a challenge in the clinical research community. At Advarra, we are committed to understanding, advocating for, and investing in increasing diversity and access in the industry. At our 2021 Virtual Spring Onsemble Conference, Tesheia Harris, Director of Clinical Research, Yale School of Medicine and Deputy Director and Chief Operating Officer, Yale Center for Clinical Investigation, Peyton Howell, President, Commercial and Consulting and Chief Commercial & Strategy Officer, Parexel, and Melissa Opraseuth, Chief Operating Officer, par8o, Inc., brought valuable insight on this topic in their panel discussion, Improving Access to Research for Diverse and Underserved Populations. Sharing their unique perspectives, each discussed barriers and strategies for improving access to research for diverse and underserved populations. The discussion presented approaches ranging from grassroots community efforts to enterprise-wide initiatives spanning large research sites and CROs.
The Consequence of Inaccessible Research for Diverse and Underrepresented Population
Understanding this vital, pervasive issue can enable sponsors, sites, CROs, and vendors to make clinical research more inclusive for all. If the ultimate goal of a trial is to get an eligible treatment to market, failing to accrue a diverse population throughout the protocol can ultimately lead to limited efficacy of the treatment. Trial findings may be skewed or misunderstood, as results will reflect a homogenous population. Since findings are limited to a very specific demographic, this stifles a researcher’s ability to find better treatments for diseases that disproportionally impact diverse populations as well. While there may be guidance from governing bodies and regulators such as the Food & Drug Administration (FDA), and policies and guidelines outlined by the National Institutes of Health (NIH), the research community must also advocate for and drive diversity and inclusion practices themselves in order to drive change in the clinical research industry.
Who is Currently Underrepresented in Clinical Research?
Before we dive into factors affecting underserved populations, we need to define the demographic and their relationship with clinical research. Reports indicate the African American community made up 12.4% of the overall population, and the Hispanic community accounted for 15%. [1] When measuring their involvement in clinical research, only 5% of the African American community was represented in studies. For the Hispanic population, the percentage dropped to merely 1%. [2]
Beyond racial representation in clinical trials, researchers need to look at factors such as:
- Geographic access to a clinical trial: How are we effectively recruiting populations that aren’t within city limits of their institution?
- Age of participants: Is our eligibility criteria excluding the elderly from research?
- Health insurance status: How does our population’s health insurance status and access to healthcare impact overall understanding and ability to participate in a trial?
 Strategies to Improve Accessibility
Strategies to Improve Accessibility
As the industry continues to grow, we need to take action to ensure inclusivity in research to support the safety, efficacy, and methodology of our patients and trials alike. While no one stakeholder group in the clinical research community can systematically increase trial accessibility, each can use their expertise and investment to impact accessibility throughout the clinical trial life cycle. Each strategy will outline the key barriers addressed, essential stakeholders and their roles, as well as lessons learned from those putting those strategies to practice today.
The trial protocol is the source for any conduct, expectations, and measurement of efficacy. When looking for a more diverse and inclusive outcome, build the necessary infrastructure at the foundation: the design of the trial.
Establish goals early: When working with a sponsor, both CRO and site leadership should advocate and set goals for more diverse accrual and outreach. By establishing metrics and key performance indicators (KPIs) early on, all stakeholders can buy into the resources and efforts necessary to achieve them. This also establishes a precedent for future trials, allowing for a data-driven approach to solidify efforts proven to work, while adjusting those needing refinement. Goals could include specific dollar amounts allocated to recruitment efforts in underserved communities, number of sites or physicians engaged in a specific region, or matching enrollment demographics with the epidemiology of the disease.
Evaluate eligibility criteria: When attempting to decrease activation timelines, many studies carry over inclusion and exclusion criteria. Instead, dictate criteria specifically for each protocol, while examining and increasing inclusivity wherever possible. For example, “A study audited 226 clinical research proposals recording exclusion of patients based on an arbitrary upper age limit and found that a significant proportion (13.7%) of clinical trials excluded patients based arbitrarily on an upper age limit.”[3] Expanded eligibility criteria also increases a participant pool and a site’s ability to quickly accrue participants to the study, therefore decreasing activation times while increasing research accessibility to underserved populations.
Gather participant feedback and implement into future studies: A great way to increase patient-centric practices into a study is to gather feedback from former study participants and incorporate it into future study design. Learn how they successfully engaged with your study and find ways to recreate those efforts across future protocols. Discuss challenges related to participation and retention by asking participants how they feel those barriers could be removed.
Design and conduct decentralized or hybrid trials: Decentralization improves participant access and experience, and reduces costs across a trial.[4] By advocating for decentralized trials to become a more normal practice at institutions, researchers can reach more participants. Currently, 70% of potential participants live more than two hours away from a site.[5] Eliminating the need for consistent in-person checkups empowers participants to complete the trial from the comfort of their own home, reaching those who may need care the most.
Conclusion
While it may be challenging, understanding how to improve access for diverse and underserved populations in research will prove to be rewarding over and over. Taking the time to build relationships with community leaders, understanding what aspects of a trial can be more patient-centric and inclusive, and learning how to communicate solutions across the industry are just a few first steps institutions and life sciences companies can make to increase access to clinical research.
Download the full whitepaper from Advarra here.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3222419/#:~:text=A%20recent%20report%20indicates%20that,15%25%20of%20those%20minority%20participants
- https://www.fda.gov/media/135337/download
- https://pubmed.ncbi.nlm.nih.gov/23240288/
- https://www.pharmaceutical-technology.com/surveys/industry-expects-dct-costs-to-be-lower-compared-with-traditional-trials-survey/
- https://www2.deloitte.com/us/en/insights/industry/life-sciences/digital-research-and-development-clinical-strategy.html
ACRO Update
 Risk-based Monitoring in Clinical Trials: Past, Present & Future
Risk-based Monitoring in Clinical Trials: Past, Present & Future
This is an abridged version of ACRO’s April 2021 article. Full version available here: https://www.acrohealth.org/rbqmlandscape/
Clinical trial management is a complex endeavor requiring careful planning, compliance with regulations, and coordination between multiple stakeholders including sponsors, investigators, contract research organizations (CROs), and clinical trial sites. Risk-based monitoring (RBM) of clinical trials has emerged as a more targeted, strategic approach that takes advantage of increased connectivity and advances in data analytics. RBM streamlines and optimizes error detection, which may facilitate the replacement of some or all on-site monitoring visits. The aim of RBM is to focus monitoring on those trial processes most likely to affect patient safety and data quality, often using real-time analytics, so that investigators can more quickly and effectively mitigate risks or address errors before they compromise trial quality.
RBM is an important component of a larger framework known as risk-based quality management (RBQM), defined in a 2013 European Medicines Agency (EMA) reflection paper as “a systematic process put in place to identify, assess, control, communicate and review the risks associated with the clinical trial during its lifecycle” [1–3]. Compared with source data verification (SDV), source document review (SDR), and other forms of monitoring focused on past events, RBM has a stronger focus on the present and future, particularly when it includes real-time monitoring and predictive modeling [4]. RBQM is a holistic, quality-management, systems-based approach to trial implementation, and RBM as a monitoring strategy is an integral part of that approach [2].
The key components of RBQM include:
- Initial Cross-functional Risk Assessment—Involves multiple stakeholders and identifies critical-to-quality (critical data and critical process) risks across the entire trial lifecycle as well as mitigation strategies, which will inform project plans.
- Ongoing Cross-functional Risk Assessment—A continuous process of revisiting and adjusting the initial risk assessment and planned mitigations as the trial proceeds based on incoming data and any new developments within or outside of the trial that could affect quality.
- Quality Tolerance Limits (QTLs)—Pre-determined limits for specific trial parameters that, when reached, a signal that further evaluation is needed to determine if action is warranted.
- Key Risk Indicators (KRIs)—Metrics used to assess site performance, either compared to other sites or to establish value.
- Centralized Monitoring—The remote review of aggregated electronic data, including data analysis.
- Off-Site/Remote-site Monitoring—Replacement of some or all on-site monitoring visits with remote-site monitoring visits, where and when allowed by regulatory authorities. When monitoring remotely, a targeted and/or triggered review of documents and data is used.
- Reduced SDV—Shift from 100% SDV to more targeted monitoring.
- Reduced SDR—Shift from 100% SDR to more targeted monitoring.
As a relatively mature concept, RBM offers established benefits to trial execution, including enhanced effectiveness of monitoring, increased overall trial quality, greater efficiency, improved patient safety, and better overall value [3,5,6]. One major advantage of RBM is its universal application to any phase trial and essentially any type of clinical study.
To shed light on the state of RBM adoption and implementation, the Association of Clinical Research Organizations (ACRO)—a trade association of CROs and technology companies—conducted a landscape survey of RBM use in clinical trials ongoing at the end of 2019 that was managed by several of its member companies. After COVID-19 emerged as a worldwide threat in early 2020, ACRO then gathered additional data from January–June 2020 to determine the impact of the pandemic on trial management, with a specific focus on monitoring. Here, we present both datasets, discussing the insights gained from them into the past and present use of RBM and how changes in trial practices during the pandemic could help shape the future of clinical trial monitoring.
Landscape Data Results
RBM Landscape Data
The landscape survey of RBM implementation during 2019 included 6,513 clinical trials managed by 7 of ACRO’s CRO member companies. Of the included trials, 47% had at least 1 of the 8 RBQM components (listed above), while 53% had more traditional trial management.
Figure 1. 2019 Landscape of Implementation of RBM/RBQM Component Implementation in Clinical Trials.
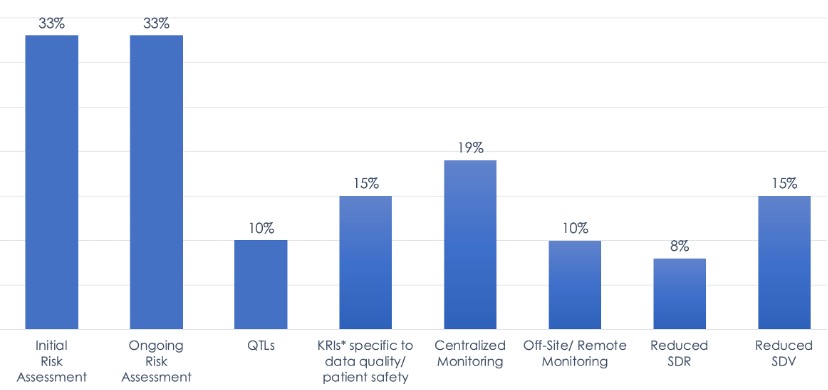
Figure 2. Implementation of RBM/RBQM Components in Combination.
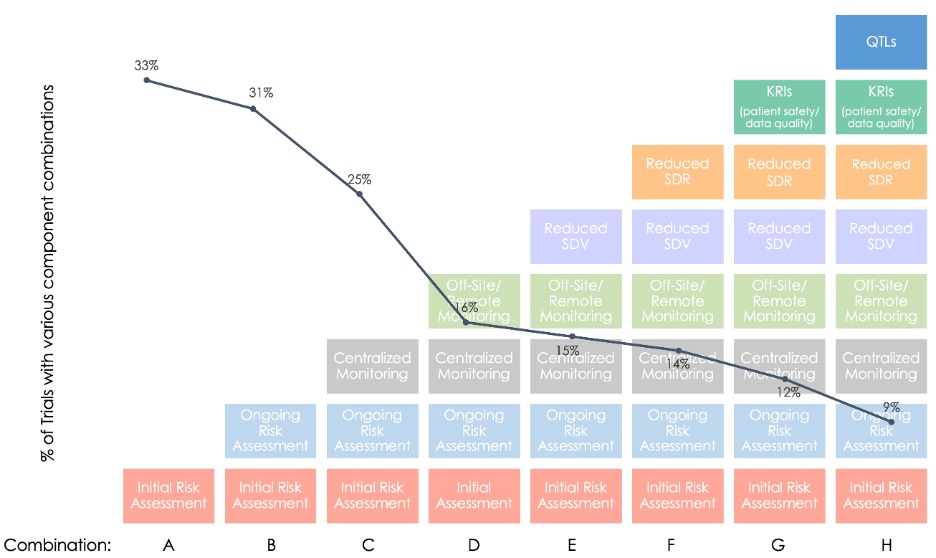
Looking at the percentage of trials with specific combinations of RBM/RBQM components (Combinations A–H in Figure 2), it is clear most trials employed neither a “holistic” RBQM approach nor a full-RBM approach.
Taken together, the RBM landscape data show that industry adoption is less widespread than expected and implementation is rather piecemeal, with few studies incorporating all five RBM components. These findings also provide a benchmark to better assess future changes in RBM uptake, particularly in situations where trial protocols and regular monitoring practices have been disrupted.
Impact of the COVID-19 Pandemic on Clinical Trial Monitoring
On March 11, 2020, the World Health Organization (WHO) declared the COVID-19 outbreaks spreading across the globe to be a pandemic. This unprecedented worldwide disruption presented major challenges in clinical trial management by forcing companies to rely mainly on remote and centralized monitoring due to site closures and stay-at-home orders. At the same time, the pandemic created something of a “natural experiment,” allowing ACRO to collect early data on the impact of this shift in trial monitoring to complement the larger-scale RBM landscape dataset.
Figure 3. Increased Remote Monitoring Visits and RBM in Response to the COVID-19 Pandemic.
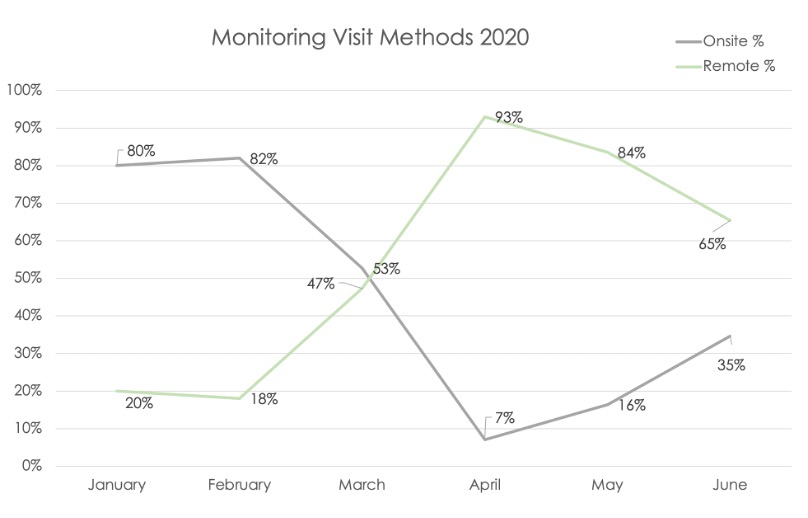
Data from 3 member companies covering trials from January–June 2020 showed remote-site monitoring increased and on-site monitoring decreased at the peak of the pandemic in April compared with the pre-pandemic baseline in February. These trends began to reverse themselves post-peak, but the percentage of remote-site monitoring visits was still markedly higher in June compared to the baseline percentage.
Figure 4. Clinical Trial Protocol Deviations Detected by RBM During the COVID-19 Pandemic.
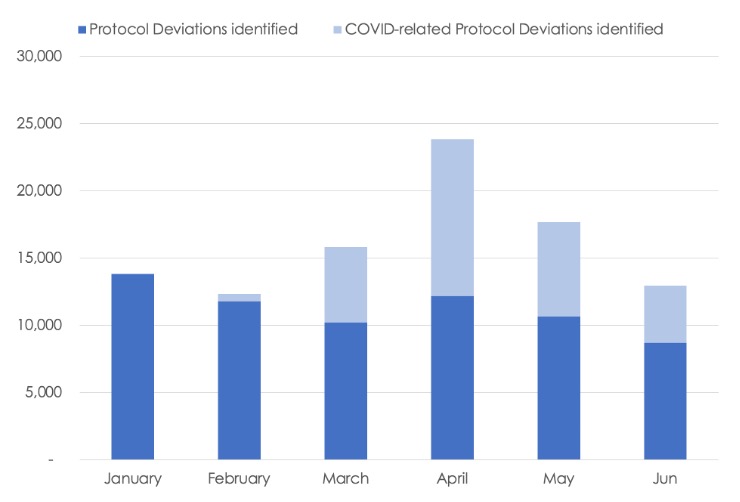 Remote site monitoring effectively captured protocol deviations as the pandemic evolved, even during the peak in April 2020 when there was little or no physical access to most trial sites. A corresponding peak in COVID-related protocol deviations was also seen that month, declining over time through June, but remaining above the pre-pandemic levels. Notably, the total non-COVID protocol deviations detected each month from March to May were similar to the February baseline, even as the percentage of remote-site monitoring visits increased from 18% in February to a high of 93% in April. This suggests that the rapid shift in monitoring methods allowed for sufficient oversight and monitoring continuity, lending confidence in data quality and patient safety.
Remote site monitoring effectively captured protocol deviations as the pandemic evolved, even during the peak in April 2020 when there was little or no physical access to most trial sites. A corresponding peak in COVID-related protocol deviations was also seen that month, declining over time through June, but remaining above the pre-pandemic levels. Notably, the total non-COVID protocol deviations detected each month from March to May were similar to the February baseline, even as the percentage of remote-site monitoring visits increased from 18% in February to a high of 93% in April. This suggests that the rapid shift in monitoring methods allowed for sufficient oversight and monitoring continuity, lending confidence in data quality and patient safety.
Discussion
Overall, execution of RBM is rather piecemeal, with the individual RBM components being used in 8%–19% of trials and very few trials executing a full-RBM approach. This inconsistent implementation is also seen for the RBQM components critical to the success of RBM, with initial and ongoing risk assessments each implemented in less than half of trials and not always together in the same trial. Not surprisingly, given the poor uptake of RBM, the use of holistic RBQM is quite rare, meaning that the full potential of RBM to enhance trial quality—by more efficiently detecting errors compromising patient safety and data validity so that their impact can be mitigated—is not yet being realized.
One reason often cited for the incomplete adoption and partial implementation of RBM is a hesitance on the part of trial sponsors and CROs to reduce the amount of SDR/SDV in favor of a more targeted approach. Other explanations for slow RBM adoption are lack of familiarity with different RBM practices, misconceptions that it might not fit into all studies, the complexity of implementing these practices, logistical barriers, the need for new and unfamiliar technology, and an incorrect assumption that RBM monitoring data is less likely to satisfy regulators.
Many of these challenges can be addressed by educating study sponsors and personnel on RBM implementation and the regulatory landscape. At the same time, stronger, more specific regulator guidance and alignment within regulatory agencies is needed, particularly when executing RBM as part of a more holistic end-to-end RBQM framework.
Conclusion
The RBM landscape survey showed that RBM adoption before the COVID-19 pandemic was not as widespread as expected, despite the proven benefits and clear potential of this approach. In addition, few trials implement more than a few of the eight RBM/RBQM components, meaning the full potential of RBM as a vital part of a broader trial management framework is far from being realized. What is clear from the rapid shift from on-site to remote-site monitoring for most clinical trials during the pandemic is that transitioning to an RBM approach without diminishing monitoring effectiveness is possible, even in difficult circumstances.
The current findings and a wealth of practical experience support the uptake of RBM and, potentially, a shift to RBQM. We believe the industry will continue to lean into greater adoption of off-site/remote-site monitoring and other RBM practices in a post-pandemic environment. To facilitate this, companies involved in clinical trial research are encouraged to share their real-world experiences with RBM implementation pre- and mid-pandemic, both the successes and the lessons learned. ACRO will continue gathering data on trial monitoring practices through the pandemic and after it has ended, to share our findings with the larger clinical research community. We further encourage the industry at large to continue to advance best practices and promote the adoption of RBM.
This is an abridged version of ACRO’s April 2021 article. Full version available here: https://www.acrohealth.org/rbqmlandscape/
References
- ACRO. Establishing Risk-Based Monitoring within a Quality-Based System as “Best Practice” for Clinical Studies – ACRO [Internet]. 2019 [cited 2020 Oct 8]. Available from: https://www.acrohealth.org/rbqm-report/
- ACRO.Risk-Basedd Quality Management (RBQM) – A Collaborative Approach toHolistic Clinical Trial Oversight [Internet]. 2019 [cited 2020 Oct 8]. Available from: https://www.acrohealth.org/oversight-rbqm-paper/
- EMA. Reflection paper onrisk-basedd quality management in clinical trials [Internet]. 2013. Available from:https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-risk-based-quality-management-clinical-trials_en.pdf
- Rosenberg MJ. Key Considerations in the Transition to Risk-Based Monitoring. Ther Innov Regul Sci. 2014 Jul;48(4):428–35.
- Brosteanu O, Houben P, Ihrig K, Ohmann C, Paulus U, Pfistner B, et al. Risk analysisand risk adapted on-site monitoring non commercial clinical trials. Clin Trials Lond 2009 Dec;6(6):585–96.
- Oversight of Clinical Investigations — A Risk-Based Approach to Monitoring [Internet]. FDA; 2013 [cited 2020 Oct 8]. Available from: https://www.fda.gov/regulatory-information/search-fda-
Site Buzz
Tracking Key Performance Indicators and Site Profitability
 As sites continue to look for opportunities to become more sustainably profitable, understanding how to identify and track key performance indicators (KPIs) has been a challenge for many. Tracking KPIs is a way for sites to showcase their value to sponsors and attract the best studies, but it can also create more transparency so sponsors and CROs have a better understanding of site operations. Sites in the SCRS online community have shared their desire to better understand what metrics are important to track and how to do so effectively.
As sites continue to look for opportunities to become more sustainably profitable, understanding how to identify and track key performance indicators (KPIs) has been a challenge for many. Tracking KPIs is a way for sites to showcase their value to sponsors and attract the best studies, but it can also create more transparency so sponsors and CROs have a better understanding of site operations. Sites in the SCRS online community have shared their desire to better understand what metrics are important to track and how to do so effectively.
Calculating profitability can look at a wide variety of metrics from human resources to financials, and some may be more important than others to sites, sponsors, or CROs. However, there may be a trade-off between the time involved for sites to measure KPIs and the value of tracking those metrics in the first place. There are many variables to consider and it can be a daunting task, especially for already busy sites. Fortunately, data can usually be extracted from a number of sources such as CTMS, CRM, accounting systems, or human resource information systems. Sites can start by going through current systems being used and seeing what capabilities are for each tool. With a number of options for technology and tools available, tracking data and KPIs can be much easier and reduce the burden on sites to track these indicators.
Study KPIs to track include enrollment percentage, retainment percentage, contract size, contract to revenue time, and duration of each study. Human resources metrics might consider employee recruitment time, how many hours it takes to train employees, staff retention rates and turnover, wage costs, and payroll/benefits costs as a percentage of total revenue. Business development KPIs may include the number of studies awarded and budgeted, outstanding proposals, lost sales and cancellations, cost of goods sold, or monthly revenue per employee. Some financial KPIs look at the age of account receivables, receivable or inventory turnover, profit margin, and working capital. Examples of clinical operations metrics would be the total number of active studies, screening visits, and average number of visits per day.
Another research site representative in the community shared that it’s important to track the percentage of time your site staff is seeing patients: “There’s a lot that goes into this metric with many factors that need to be considered before making management decisions. This metric correlates with revenue, quality, morale. In short – you look at the total number of available time in a day/month you have to see patients and compare it to how much time was actually spent seeing patients. You should be able to calculate this using your CTMS. To give you an idea, our sweet spot is 40-45% which means in a given month we spent 40% of our time seeing patients. We all know that the bulk of time needed to do a study is all the ancillary work. When that number creeps up over 50% you can really feel it in the office. Tensions can run high, staff is clearly working much harder to get [everything] done to the point where they are playing catch-up. Quality can slip. Sure you’re generating more revenue via patient visits but this metric can help you assess the need for more help. Alternatively, if your percentage of time with patients dips down below 30% you will notice it through idle staff and less revenue. Again, you need to hold those accountable for business productivity.”
When tracking KPI data provides added benefits, such as opportunities for increased funding, making hiring decisions, forecasting revenue, or understanding bottlenecks in the clinical operations processes, it should be prioritized. That’s why it’s critical for sites to start by understanding what metrics are most important to stakeholders and how the data will be used. If sites are taking time to collect certain metrics and track KPIs, the data also needs to be reviewed, analyzed, and shared with necessary stakeholders as well. When possible, sites should assign one person or a team of people who will manage the tracking of data and KPIs. Sponsors, CROs and site study staff can work together to identify what’s most important to track and how to understand the data being collected to make informed decisions that can impact both trial and site profitability.
About SCRS
Founded in 2012, SCRS is a global trade organization that unifies the voice of the clinical research site community to create greater site sustainability. Representing over 9,500 sites in 47 countries, SCRS membership provides sites with a community dedicated to advocacy, education, connectivity and mentorship. SCRS is an influential voice for sites and an active partner in industry-wide initiatives and dialogues focused on improving the clinical research enterprise. Our Voice. Our Community. Your Success. Join the community.

