January 2021
Welcome to the January issue of InFocus, where we provide insights and solutions to help sites and other stakeholders ensure site sustainability.
SCRS Current
The Team at SCRS has never been more excited and optimistic for a new year as we approach 2021. We have a full slate of Summits and events scheduled, as well as new innovations and ideas for our research site community. Registration for all Summits is now open on the website: the Global Oncology Site Solutions Summit, the European Site Solutions Summit, the Asia, Australia & New Zealand Site Solutions Summit, and the Global Site Solutions Summit.
The first Summit on the schedule is the Global Oncology Site Solutions Summit. Though we plan to return to in-person events later in the year, this Summit has been planned as a virtual event. Through the support of SCRS partners, we are able to offer a complimentary attendance option for sites and bring in unique content from industry partners and industry associations.
Keep an eye on the website and your inboxes for other events. The webinar calendar is quickly filling up and will provide at least 36 presentations with CEU’s this year. Sites NOW, which started at the 2020 SCRS Global Site Solutions Summit, has a full calendar that will continue through to the next Global Summit.
As we continue to work with the disruptions imposed by COVID-19, SCRS has been advocating for sites. One issue that has come up is that certain categories of sites, including those that are conducting research studies with COVID-19 patients, have not necessarily been considered in the COVID-19 vaccination prioritization. In the US, SCRS has partnered with ACRO and ACRP to advocate for sites to state leadership officials in government and public health.
We have several other initiatives ongoing, including working to end payment holdbacks, Diversity in Clinical Trials, and the Digital Innovation Initiative to represent the needs and voice of the site community as the industry develops new technologies. In addition, SCRS COO Allyson Small is a co-chair of the #NoGoingBack movement, encouraging everyone in industry to commit to preserving the progress in clinical research that was made during 2020 despite the many challenges that arose.
As discussed in the Metrics that Matter article in this newsletter, we are seeing progress in metrics that SCRS has documented for years. The initiatives and projects sites and industry have worked on together are bringing real improvements in how clinical trials are conducted. This year will call upon us to be more nimble and innovative than ever, and we look forward to seeing that reflected in the report we will give at the 2021 SCRS Global Site Solutions Summit.
Metrics that Matter
Site Selection Communication
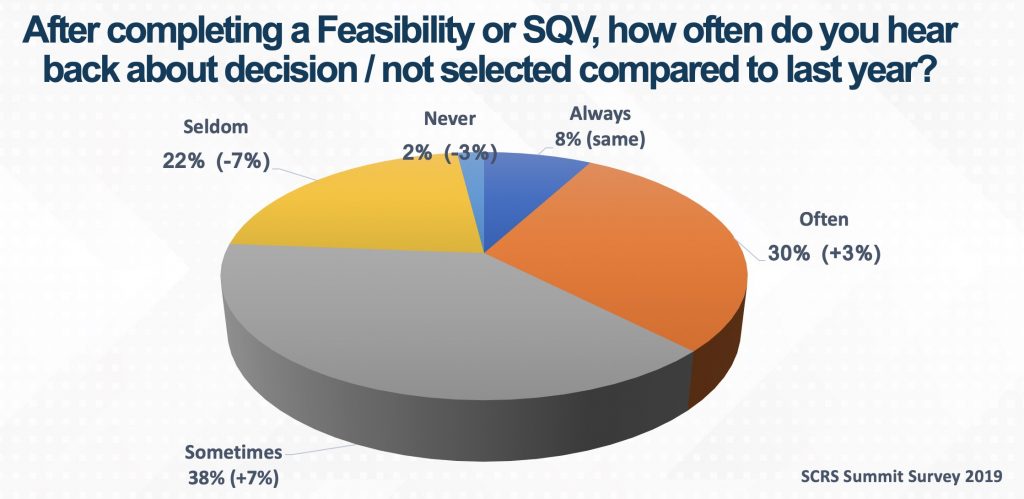 Since its inception, the SCRS Site Landscape survey has invited sites to report how often they receive a decision from a sponsor or CRO after a feasibility questionnaire has been completed or a site qualification visit (SQV) has occurred. Their responses track the improvement of communication between sponsors, CROs and sites. In 2020, only 24% of sites stated that they seldom or never received feedback. This is 10% lower than the year before, indicating that more sites are receiving a response to their feasibility questionnaire or SQV.
Since its inception, the SCRS Site Landscape survey has invited sites to report how often they receive a decision from a sponsor or CRO after a feasibility questionnaire has been completed or a site qualification visit (SQV) has occurred. Their responses track the improvement of communication between sponsors, CROs and sites. In 2020, only 24% of sites stated that they seldom or never received feedback. This is 10% lower than the year before, indicating that more sites are receiving a response to their feasibility questionnaire or SQV.
The transformation of clinical trials to a more virtually enabled model in 2020 has enabled sites to quickly respond to clinical study opportunities. The more efficiently both parties are able to respond to study opportunities, the more sites, sponsors and CROs can meet the needs of patients waiting for product development. Sites waiting for studies, with no response, might lose the opportunity to pivot to other studies. Sponsors and CROs that don’t share site selection information may lose out on future trials with sites.
With 76% of sites indicating that they sometimes, often or always receive feedback, critical communication about site selection has improved significantly. This year, SCRS challenges our sites and partners to continue the positive trend. Sites are encouraged to contact sponsors and CROs and follow up on feasibility questionnaires and SQVs. Inquire about the status of site startup by scheduling tasks to initiate these check-ins and be proactive in ascertaining when you should expect to receive an update or response.
We ask our sponsor and CRO partners to proactively keep sites informed of site selection and site startup activities. Include in your processes the necessary steps to provide timelines and updates to sites as to when they can expect to receive communication related to their feasibility work. As the world strives to bring this pandemic under control, the backlog of studies is going to provide sites with more options. Keeping sites informed of their status with your study is the most effective way to recruit the sites you have determined are the best match.
We look forward to collecting an even more improved metric at the end of 2021!
SCRS Connects
Nicole Osborn and Community Engagement

At age 22, Nicole “Nikki” Osborn was a study coordinator for a physician who did research part time. A sponsor representative pulled her aside at an investigator meeting and suggested she open her own site. Ms. Osborn took the opportunity and opened her first research site.
That site led to co-founding Meridian Clinical Research, which has now grown to 24 sites with more than 300 employees. In the 20 years since she opened that first site, Ms. Osborn has seen a lot of changes in how the public perceives clinical research. “When I started, we advertised in newspapers and had to explain what clinical trials were,” she remembers. “Trial information and opportunities are now more accessible for potential patients, and people are more informed about the role of clinical research today.”
Instead of telling people what research is, the recruitment challenge has shifted to being a trusted member of the community. To build that community relationship, Ms. Osborn established a charitable program called “strength in numbers.” The business contributes to a fund based on the number of patients enrolled in studies, and employees determine where money should go in their community. “We support everything from charity walks to kids’ baseball teams; the kind of groups and activities that strengthen communities,” explained Ms. Osborn. “We also support individuals facing personal battles, such as local families raising money for unexpected medical expenses.”
These projects build relationships directly and expand the sites’ social media footprint. “Instead of us reaching out to patients in newspaper ads, patients search for us online,” explained Ms. Osborn. “The patient is more empowered than ever. We’re committed to making clinical trials more visible and accessible to potential patients.”
In a competitive resourcing environment, the program also helps with employee retention and engagement. “We want to empower Meridian employees to make a positive difference in their community beyond the clinic,” said Ms. Osborn. “They’re entrenched in the communities we serve — we trust them to maximize local charitable efforts.”
After two decades of change in research, the COVID-19 pandemic brought new challenges. “We were catapulted into virtual visits,” Ms. Osborn said. “We had the technology in place. Our staff adapted quickly. They made patients comfortable. For years, the industry has trended toward more virtualized trials. Suddenly, it became our new reality.”
Looking ahead, Ms. Osborn’s concern is that these changes not be reversed. “I don’t see the industry regressing. SCRS is involved with #NoGoingBack and I support that program,” said Ms. Osborn. “We did something remarkable together this year. We must build on that progress.”
Member Spotlight
What’s Happening in Oncology
As we approach our 2021 Global Oncology Site Solutions Summit – Virtual Experience, the membership team wants to be sure you are aware of two of our latest oncology-focused benefits. They were developed with our partners to meet the unique needs of members who participate in oncology trials and help them reach the next level operationally.
 One such tool is the response evaluation criteria in solid tumors (RECIST) training modules. This common methodology for evaluating solid tumors is used by sponsors and CROs in oncology trials, and this training course is generally accepted as the necessary training for this process. The training modules consist of both the RECIST 1.1 version for assessing tumor burden over time and the iRECIST training which is modified for immunotherapy. For those of you with staff who need to take this training, we encourage you to participate in these modules.
One such tool is the response evaluation criteria in solid tumors (RECIST) training modules. This common methodology for evaluating solid tumors is used by sponsors and CROs in oncology trials, and this training course is generally accepted as the necessary training for this process. The training modules consist of both the RECIST 1.1 version for assessing tumor burden over time and the iRECIST training which is modified for immunotherapy. For those of you with staff who need to take this training, we encourage you to participate in these modules.
SCRS’ online communities are a valuable resource where our members can engage more directly with their other site peers and members. In this virtual world we live in, this connection and casual method for asking questions and gaining new insights is truly invaluable and important to our community. These communities are a place to ask questions and share knowledge in a meaningful way. We have an oncology-specific online community, specifically for our sites that participate in oncology research. Signing up is simple: just send a request to onlinecommunity@myscrs.org. Make sure you read the community code of conduct first.
are a valuable resource where our members can engage more directly with their other site peers and members. In this virtual world we live in, this connection and casual method for asking questions and gaining new insights is truly invaluable and important to our community. These communities are a place to ask questions and share knowledge in a meaningful way. We have an oncology-specific online community, specifically for our sites that participate in oncology research. Signing up is simple: just send a request to onlinecommunity@myscrs.org. Make sure you read the community code of conduct first.
These are just two benefits SCRS offers that work to support our oncology site community. Coupled with our upcoming Oncology Site Solutions Summit, they are industry-leading ways for you to stay on the cutting edge of oncology trial participation. We are very excited for the upcoming Oncology Site Solutions Summit, and can’t wait to see you all there.
TransCelerate Update
Beyond COVID-19: Modernizing Clinical Trial Conduct

The COVID-19 pandemic has challenged the way that healthcare and pharmaceutical industries deliver care to patients. Traditional methods for conducting clinical trials have been disrupted in ways never before seen. This has forced both regulators and biopharma to think progressively about how to enable and execute new methods for delivering care to patients during clinical trials. There is a rich opportunity to understand the numerous lessons learned, including both challenges and successes. Innovative thinking and modified regulatory policy have allowed for the implementation of continuity solutions to accommodate the ongoing conduct of clinical trials while maintaining subject safety and data integrity.
The implementation of continuity solutions has impacted all stakeholders involved in clinical research, including sites. These learnings can be used to inform future ways of working for industry as well as future policy by regulators.
Understandably, study sites and institutions were primarily focused on managing and maintaining participant safety during the pandemic rather than implementing new technology to keep a clinical trial running. TransCelerate saw an opportunity to help solve some of these problems.
What do we see as benefits to sites?
- Acceleration of patient-centric approaches to clinical development
- Greater understanding of innovative clinical trial methodologies that support more effective patient engagement, when compared to traditional methods
- Potential to create efficiencies from accelerating health authority acceptance of innovative clinical development methods
Modernizing Clinical Trial Conduct
Earlier this year, TransCelerate quickly mobilized the Modernizing Clinical Trial Conduct Initiative to tackle some of the issues the industry is facing. This initiative uses data and experience to develop practical guidance and solutions to further enable the successes of continuity solutions implemented during COVID-19 into future ways of working.
TransCelerate published,“Beyond COVID-19: Modernizing Clinical Trial Conduct,” a white paper that proposed the ongoing utilization of innovative continuity solutions implemented during the COVID-19 pandemic. These solutions have fulfilled the critical need for continuity of trial participant oversight, assured data integrity and maintained investigational medicinal product supply. The pandemic catalyzed the expansion and acceleration of existing continuity solutions (e.g., telemedicine) as well as the establishment of new ones (e.g., direct to patient shipping). The white paper describes broad categories of continuity solutions utilized, the challenges related to their use, and the factors that made implementation successful.
The role of technology
A 2020 SCRS site staff survey showed that staff recognize the potential benefits of using technology in clinical trials. The key potential benefits cited by site staff were greater participation in and access to clinical trials. Sites considered the distance between the participant’s home and the site and the frequency of site visits to be the biggest deterrents to clinical trial participation. These issues could be mitigated by telemedicine, home health visits, increasingly expressing dissatisfaction with current eClinical technologies as a result of disparate systems. This sentiment further supports the need for modern, adaptive, and integrated approaches to clinical trials. Importantly, it should be noted that sites perceived overall quality of study data and participant safety as challenges to decentralizing clinical trials. Sites and sponsors will need to work together to assuage these concerns as remote technologies become more commonplace.
Additional resources published include continuity solution case studies and the findings from a collaborative workshop between TransCelerate and the Association for Clinical Research Organizations’ (ACRO) CRO Forum. This September 2020 workshop explored root cause challenges and enablers of success in the use of modern methodologies in clinical research, which will be discussed below. These issues will need to be tackled and developed by distinct shareholders across the industry.
Telemedicine
For telemedicine, sites were facing a few hurdles during the pandemic. As telemedicine is still not widely adopted, there is inadequate data demonstrating the experiential impact of telemedicine. Additionally, there is a variation in telemedicine technology platforms used by sites. These variants result in different levels of patient acceptance and a learning curve on how to use the telemedicine technology.
For telemedicine to be useful, enablers of success can be implemented. First, a standardization of a telemedicine definition should be established by a standard setting organization. This can help to establish consistent baselines for adoption, technology, quality, and deployment that all stakeholders can follow, including patients.
Patients and sites should be engaged to better understand their perspectives, experiences, and preferences for telemedicine, including acceptance criteria. This, combined, with enablement of a positive culture change to start incorporating telemedicine and an allowance of flexibility and use with other solutions like home health visits, can allow for a more diverse and accommodating clinical trial experience.
Home Health Visits & Local Lab Use
As mentioned above, sites have inadequate data demonstrating the impact of home health visits and local lab use by patients and sites. There is also a lack of data demonstrating the acceptance of these visits from these two groups. Overall, the lack of data on how these and the below solutions are used is one of the most commonly cited hinderances to site staff and sponsors alike.
Enablers of success for sites might include a mapped-out patient journey (e.g., standard of care vs. home health visit assessments); a business case to support using home health visits in a hybrid mode; establishment of equivalency of home health visits to in-person visits; and development of operational manuals to ensure effective oversight and quality, among others.
Direct-to-Patient Shipping
To help sites accumulate data demonstrating the impact of direct-to-patient (DTP) shipping, potential solutions that can be enabled include the establishment of model processes for different DTP approaches (i.e., incorporating differences in geography and TA, vendor networks, available solutions); solutions in therapeutic areas with immunocompromised patient populations; elimination of dispensing-only site visits for patients; and clear instruction for patients to report adverse events in a DTP model.
Tools for success
The successful implementation of continuity solutions during the COVID-19 pandemic depends on numerous contributing factors. One overarching success factor is the desire and determination of regulators, sponsors, sites, and vendors to minimize the disruption of treatment and care for participants. Successful stakeholders possess the following attributes which contribute to the overall success of these continuity solutions:
- Openness to modified ways of working to enhance infection control and promote more virtual, remote, or decentralized methods for conducting trials
- Willingness to be creative and/or flexible without one-size-fits-all solutions (e.g., allowing some participants/sites to have in-clinic visits and others to have telemedicine or home health visits).
- Desire to work together and prevent increased burdens from becoming too much for any one group (e.g., willingness to look for ways to reduce site burden)
- Recognition of the potential benefits and consideration of the work required to implement continuity solutions as an investment towards a better future
Other success factors relate to actions rather than mindset. Some of these success factors are a result of industry’s activity prior to the COVID-19 pandemic:
- Ongoing research/consideration of digital data collection tools and other technology platforms to modernize data collection and reduce the study monitoring burden
- Ongoing research/consideration of other possible continuity solutions (regardless of disruption) to reduce the burden for clinical trial participation
- Established use of Risk Based Monitoring to identify the appropriate approach for monitoring critical data so that SDV or source data review (SDR) via remote monitoring can focus on key issues
Presumably, refinement of and prospective planning for continuity solutions will result in better clinical trials which may be modernized, flexible, decentralized, and patient-centric. Full realization of modern clinical trials will require the support of key stakeholders including participants and sites. While supporting data is still needed and being generated, early input from both stakeholders point to a desire for modernizing clinical trials via prolonged use of the referenced continuity solutions.
Site Buzz
#NoGoingBack on Flexibility
 If there is one thing 2020 has taught us, it’s that a “one-size-fits-all” model for study management and execution isn’t required. We have proven that there is flexibility in what the regulations and our protocols require. These new options for executing clinical trials have the potential to set up our sites and patients for future success.
If there is one thing 2020 has taught us, it’s that a “one-size-fits-all” model for study management and execution isn’t required. We have proven that there is flexibility in what the regulations and our protocols require. These new options for executing clinical trials have the potential to set up our sites and patients for future success.
Sites in our online community have discussed some legacy rigidity that harms sites and is likely not necessary going forward. One rigid practice is requiring all sites involved in a study to close at the same time. Sites are sometimes required by sponsors and CROs to keep a study open for years even though the study is no longer active. When a site is in this situation, their hold back is often retained as final queries and data lock are not issued until the entire study goes through the final closeout process. At that point, the site staff may not remember the patients or may no longer be employed by the site.
What would be required to allow sites to close when their last patient concludes their participation in the study? Resourcing can become an issue at the site level with lingering trial activities, and there are losses to data quality when queries come quite some time—as long as years—after they last saw the patient.
The use of home health nurses on a study is becoming subject to new rigidity that we can address before it becomes a larger issue. Home health nurses have been key to the flexibility that has allowed studies to continue with fewer exposures for patients and study staff. However, this has led to home health nurse agencies being contracted directly by sponsors and CROs for all sites. Some sites have established relationships with home health nurse agencies in their geographical area and are able to work efficiently within this existing relationship. Allowing both options—using a local agency or using the centralized agency selected for the study—is critical to meeting the needs of a majority of sites and conforming to their practices.
Studies that require specialized providers for specific portions of the study such as ophthalmologists, psychometrists, and neurologists have traditionally relied on sites to secure and delegate those roles locally. This has become more difficult as these ancillary providers focus on the needs of their established patients during the pandemic. As a result, sponsors and CROs have started to develop national networks to provide these research services. The flexibility to involve a specialized provider selected by the study team is allowing more sites to respond to more demanding studies.
By building on the flexibility developed in 2020 and incorporating the principles of #NoGoingBack, our sponsors, CROs and sites can provide the most they have to offer for study success in a way that supports their normal site operations. Sites are hopeful for even more flexibility moving forward. We will have to work together to ensure that the flexible options that became more prevalent in 2020 aren’t hindered by additional rigidity as they are incorporated into standard study processes.
SCRS Sites NOW
Building a Better Clinical Trial Budget Model
 Molly Downhour, MHA BSN NEA-BC OCN CCRC
Molly Downhour, MHA BSN NEA-BC OCN CCRC
National Director Clinical Research
Medix
Clinical research is a dynamic industry built around continuous learning from the successes and failures of process and science. As the clinical research industry has responded to the COVID-19 pandemic, we have found new urgency in identifying and overcoming barriers to successful clinical trial execution. One business-driven success factor that routinely comes under scrutiny is clinical research budgets and the planning or flexibility in their performance for all involved parties.
There are endless webinars, white papers, training modules, vendors, consultants, and conferences all seeking to “improve” clinical trial budgets for clinical research sites. Nicholas Slack, WCG Clinical Executive Vice President and Chief Commercial Officer, recently shared that, on average, sites subsidize approximately 38% of their clinical research costs from other operations (Ramsey, 2020). According to SCRS’s 2020 Landscape Survey, 53% of sites have three months or less of operating cash available. This number reflects a steady decline from 2018, when 64% of sites had three months of operating cash (SCRS Landscape Survey, 2020). This opens the question, if we have all of these resources to help sites improve their budgets, why are they continuing to struggle?
Let’s get the obvious culprits out of the way: quarterly payment terms, hidden budget items, and holdbacks. The industry has done a great job of changing these traditional models, especially in response to the COVID-19 pandemic. Sites want monthly payments, no holdbacks, and transparency in regard to acknowledging expenses that are identified after the budget is executed. For example, expenses may be identified from a lab processing manual that is not available until the site initiation visit. At this point, a budget amendment may be needed, and sponsors and clinical research organizations (CROs) have come to accept that reality. These issues are not the primary problem.
The main issue causing financial strain in site budgets is the use of commercial grant databases that set the standard for fair market value for sponsors and CROs. Sites’ clinical trial budgets are compared to these tools, but sites are unable to evaluate these tools for themselves. This is the system we need to fix, as it is inherently flawed. These databases fail to account for contexts where budgets are below market value, and therefore create standards that are not sustainable for sites. Some of these contexts include:
- New sites with “loss leader” budgets trying to get their foot in the door.
- Inexperienced sites that don’t have an adequate budget review process.
- Sites undercutting budgets for competitive advantage knowing they have philanthropic subsidies for sustainability.
- Academic medical centers that are extremely cautious about creating an income stream that will detract from their sense of mission.
- Investigators that see research more in terms of career fulfillment and less as part of their site’s bottom line.
Some additional contexts that are created by the industry itself are:
- Pressuring sites to open quickly and accept low budgets.
- Finalizing the contract before ancillary materials that are necessary to review the budget, such as the laboratory manual, are available.
An additional issue faced by hospital systems doing research is that cost sheets may change as often as quarterly. At present, there is no effective way to update study budgets to match these cost sheet changes. Sites’ best strategy is to estimate cost increases based on the anticipated length of the trial, knowing that budget amendments without a protocol amendment will likely not be a priority.
There are many other flaws in the current design process that are too specific to go into here. In short, we have a process that models budgets solely on patient activity, study startup and expenses that can be invoiced (excluding all the non-billable work). This may not be the best way to model the budget, but as an industry we haven’t developed anything better.
Sponsors and CROs then add a layer of complexity to the budget process by sending out low budgets that they expect to require negotiation. The pressures of COVID-19 research have revealed this practice to be a charade, as sites report budgets that seem to have come in at a higher rate that could more quickly be finalized. A common saying in the industry is that “sponsors will not balk at sites asking for up to 20% increase from the original budget they send.” Why play this game when time is of the essence for all stakeholders – most importantly our patients?
Sponsors do need a tool to justify expenses, demonstrate fair market value, and protect themselves from perceptions of conflicts of interest or any malicious activity that could risk the integrity of the research. However, we also need to recognize that grant databases gather data from sites with varying business savvy, education, agendas, and geographic cost to do business. When sites that do not understand their own costs accept sub-standard budgets or when they are in low-cost zones, their budgets go into the average of the data that all sites are held to (but cannot see).
It is clear that a working group composed of the great minds in the industry (and outside the industry) is needed to develop an alternative model that addresses not only the inaccuracy of commercialized grant databases, but also the flaws in current clinical trial budget models. Having an accurate tool for all key stakeholders to use should decrease wasted time in budget negotiations, protect all parties involved, and help keep clinical research sites from closing due to financial unsustainability.
Ramsey, L. (2020). Sites overwhelmed by cost and trial complexity, need sponsor help to bear the burden. CenterWatch. Retrieved November 25, 2020 from https://www.centerwatch.com/articles/24969-sites-overwhelmed-by-cost-and-trial-complexity-need-sponsor-help-to-bear-the-burden.
Society for Clinical Research Site
Sites NOW Supporters











































Sites NOW Participating Organizations
AbbVie | Accel | Accellacare (ICON Plc) | Advarra | AstraZeneca | Bio-Optronics | BRANY | Bristol Myers Squibb | BTC/ClinEdge Site Networks | Clearwater Cardiovascular Consultants | ClinEdge | Clinical Site Partners, LLC | Clinical Trials of Texas, Inc.(CTT) | Clinical.ly | Clinvest Research | Complion | Covance | CTMD Clinical Research | DM Clinical Research | East Coast Institute for Research | Elite Research Network | Evolution Research Group | GlaxoSmithKline | Greenphire | HCA Healthcare | ICON, plc | Janssen R&D | LMC Manna | Research Medical deScriptions | Medix | Medpace | Meridian Clinical Research | Northwell Health | OrthoIllinois | Oviedo Medical | Research | Parexel | Pfizer | Pharmaseek | PMG Research | Prime Site Research Solutions | RealTime Software Solutions | Ripple | Science Corporation | Roche | RX Trials | Sanofi | Sentral Clinical Research Services | South Broward Research | StudyKIK | SubjectWell | Suncoast Clinical Research | Syneos Health | Synergy Clinical Research | Total Clinical Trial Management | Trifecta Clinical | Veeva | Veterans Research Foundation of Pittsburgh | VirTrial | WCG | PharmaSeek
#NoGoingBack
‘No going back’ for clinical trials after COVID
(Published by Pharmaphorum, December 2020)
Trial sites have adapted swiftly to the restrictions of COVID-19, and patients have seen many knock-on benefits as a result. The next step is ensuring the industry does not regress to old ways of working once the pandemic is over, say Karen McIntyre and Allyson Small.
COVID-19 has changed everything for clinical trials – but in most cases these are changes that were well overdue.
“For years and years, the industry has debated the practicalities and safety of decentralising clinical trials, using telemedicine, and where study activities should take place,” says Karen McIntyre, executive director, global lead Catalyst Program & site relationships at Syneos Health. “We were always having these discussions, but nothing moved forward.”
When the pandemic hit, regulators around the world rapidly updated their guidelines to reflect the realities of conducting trials amidst lockdowns and social distancing mandates.
“For example, drugs are now able to be delivered directly to patients to allow for a clinical trial visit to take place remotely,” says McIntyre.
“These changes didn’t necessarily need to involve advanced technology – we just had to adapt the way we worked.”
She says that sites quickly rose to the occasion.
“We really saw sites’ commitment and dedication come to the fore. They adapted quickly and were incredibly flexible, and rapidly became confident that, yes, they can continue to work effectively and safely with patients in the new normal.”
McIntyre had first-hand experience with just how effective alternate ways of interacting with patients can be when her husband presented with shingles just days after lockdown began
“Normally, we would phone our GP surgery in the morning to try and get an appointment. In the likely event that we couldn’t get an appointment that day, we’d have to phone again the next day, and the next, until we’re finally able to see a doctor.
“This time, though, I just took a photograph of my husband, emailed it to the doctor and got a phone call 11 minutes later to say that there was a prescription at the chemist.”
Now, she says, the industry is wondering why it was ever afraid of change.
“These approaches have all worked, and they’ve made clinical trials much more inclusive. Everything has changed, and I think that there’s no going back.”
Diversity and access
One change McIntyre has seen is that the industry has stopped focusing on the challenges of implementing new clinical trial technologies and is now looking at what sites already have and what they need going forward.
She adds, though, that the industry has to be wary of imposing technology on sites.
“One size won’t fit all. Technology that works for a large academic hospital won’t necessarily be optimal for a dedicated research site. The industry also needs to think long-term – don’t make technology for one specific study, but technology that can be reused for multiple trials.”
A huge boon of adaptations like remote monitoring is they make patient access to trials wider than ever before.
“Most people live hours away from their nearest clinical research centre – and for people with rare diseases it might even be in another country,” says Allyson Small, COO at the Society for Clinical Research Sites. “If you are working full time, or have a family to look after, that means you’re prohibited from being included in many studies.
“Having flexibility therefore means that clinical studies can be much more diverse, allowing researchers to bring in patients from all over the country or even the world.”
She notes, though, that it will be important to ensure patients have as many options as possible for how they participate in trials – for example, many patients still value human interaction above anything else and are keen to get back to their trial sites. For this reason, Small and McIntyre say hybrid trials are likely to be the dominant format in the future.
Similarly, many patients also have safety concerns with remote trials, and making sure that all patients feel safe outside of a typical trial setting has been a key task for sites.
“Before COVID-19, a term that was being bandied about a lot was ‘siteless trials’ – but really you can never have a siteless trial, because you will always need clinical oversight,” says McIntyre. “It’s not just about bricks and mortar, it’s about being able to conduct these procedures safely, and sites are just as important for remote and decentralised trials as for traditional trials. I think people realise that now – I haven’t heard anyone use that terminology in months now.”
The pledge for progress
It’s too early to say whether regulators will want to keep more flexible rules in place once the risk of the pandemic is gone – especially when each regulator is different, and this has been a worldwide phenomenon. But McIntyre says the industry’s opinion on the matter couldn’t be clearer.
“Everybody you talk to will have different opinions, but overwhelmingly, the industry response has been that there is no going back.
“We have learned that we can reduce the timelines of study start-up and increase diversity of participants, and that there are multiple ways we can collect safety data and get investigation products to patients. It’s been amazing to see the collaborations that have driven that.
“We need to keep progressing and not regress back to endless discussions and debates. We’ve learned that it can work, that we can stop thinking that a clinical trial site is a place – rather, a clinical trial site is an activity.”
To this end several companies have come together to support the #NoGoingBack movement launched by Signant Health – which is asking people from the industry to pledge to keep clinical trials moving forward into this bold, patient-centric future and not let progress slip away.
The movement’s charter asks signatories to honour the lessons the industry has learned – namely that patients deserve and want flexible options for trial participation, that the industry can adapt faster than it thought it could, that collaboration between competitors is needed for acceleration, and that pharma has an “unparalleled opportunity” to make huge improvements in drug development.
“We need to keep this acceleration in technology adoption and new ways of working moving forward,” says Small, who is one of the campaign’s chairpeople. “It was amazing to see everyone, from sponsors to CROs and sites, rallying together at the beginning of the pandemic to share experience and ensure clinical trials could continue to serve patients, and we want to make sure that effort wasn’t in vain.
“If we do that, we can move away from a ‘one size fits all’ approach to trials. Every patient is different, and to have one protocol with no flexibility adds unnecessary challenges to sites.”
McIntyre adds: “So far everything we’ve done has been through protocol deviations, and now we’re asking for regulators to work with the industry to create protocols that will allow for those flexibilities and are inbuilt into the clinical trial process.”
She believes this goal is eminently possible.
“I think regulators are seeing the benefits. We’ve proven that we can do this. Never before in my career have so many people from all levels come to ask me what’s happening with the sites. That shows there’s a real drive here, and now we just need to make sure that sticks.”
About the Interviewees
 Karen McIntyre is executive director, global lead Catalyst Program & site relationships at Syneos Health. Karen brings almost three decades of industry experience in a variety of therapeutic areas including cardiovascular and metabolic disorder, women’s health, neuroscience and infectious diseases in phase II through phase IV clinical trials. With a special interest in site support Karen has been involved in the development of Site Support Management tools with the goal to improve quality, transparency and compliance across investigative sites since 2005. Karen is also an active member of National Research Ethics Committee, Scotland.
Karen McIntyre is executive director, global lead Catalyst Program & site relationships at Syneos Health. Karen brings almost three decades of industry experience in a variety of therapeutic areas including cardiovascular and metabolic disorder, women’s health, neuroscience and infectious diseases in phase II through phase IV clinical trials. With a special interest in site support Karen has been involved in the development of Site Support Management tools with the goal to improve quality, transparency and compliance across investigative sites since 2005. Karen is also an active member of National Research Ethics Committee, Scotland.
 Allyson Small is chief operating officer of the Society for Clinical Research Sites (SCRS). With nearly 20 years of experience working in the healthcare industry, Small joined the SCRS team in 2013. She plays an integral role in strategic growth, developing key partnerships, and increasing global membership by 80%. Small also oversees all development and production of four International Summits. Small is a champion for creating a voice for clinical research sites across the globe and assuring site sustainability.
Allyson Small is chief operating officer of the Society for Clinical Research Sites (SCRS). With nearly 20 years of experience working in the healthcare industry, Small joined the SCRS team in 2013. She plays an integral role in strategic growth, developing key partnerships, and increasing global membership by 80%. Small also oversees all development and production of four International Summits. Small is a champion for creating a voice for clinical research sites across the globe and assuring site sustainability.
About the Author
 George Underwood is a senior member of the pharmaphorum editorial team, having previously worked at PharmaTimes and prior to this at Pharmafocus. He is a trained journalist, with a degree from Bournemouth University and current specialisms that include R&D, digital and M&A.
George Underwood is a senior member of the pharmaphorum editorial team, having previously worked at PharmaTimes and prior to this at Pharmafocus. He is a trained journalist, with a degree from Bournemouth University and current specialisms that include R&D, digital and M&A.
About SCRS
Founded in 2012, SCRS is a global trade organization that unifies the voice of the clinical research site community to create greater site sustainability. Representing over 9,500 sites in 47 countries, SCRS membership provides sites with a community dedicated to advocacy, education, connectivity and mentorship. SCRS is an influential voice for sites and an active partner in industry-wide initiatives and dialogues focused on improving the clinical research enterprise. Our Voice. Our Community. Your Success. Join the community.
