Case Study: Building Patient Cohorts Faster With Clinical Technology
In recent years, much has been made of the proliferation and use of digital tools to enable patient identification and matching to clinical trials. But within the everyday world of a busy investigator site, how useful are these tools?
In this case study we examine a real-world, practical example of the significant time and resource savings that can be gleaned by the application of such tools.
THE CHALLENGE
COHORTS
For an observational multi-center cohort study on metastatic hormone-sensitive and castration-resistant prostate cancer, a database was created capturing patient characteristics, disease features, clinical outcomes, and healthcare utilization insights through chart review. To date, 19 centers have been included to create a national “Real World Data” registry, with the number of participating hospitals increasing annually.
19 HOSPITALS
Data for this multi-center study were previously collected manually, a time-consuming and costly task. In addition, the data captured only reflected a snapshot in time. To update insights, manually re-gathering data per hospital would be required, which limited the speed and scope of the study. A suitable alternative to this time-consuming, error-prone, and irregular process of manual data collection was sought.
THE SOLUTION
The use of digital patient-finding technology enabled the automation of the data collection process. Data could be collected simultaneously from multiple hospitals through a process that adhered to all data privacy considerations. The deployed technology was able to search through both structured and unstructured Electronic Health Record (EHR) data. Natural Language Processing algorithms (NLP), embedded in the technology, extract the medical concepts and measurements from the clinical notes and pseudonymize the data. After this, the data from various sources is harmonized and loaded into the database.
The search set-up (query) was refined and improved several times during the project, based on characteristics and symptoms of the patients already identified.
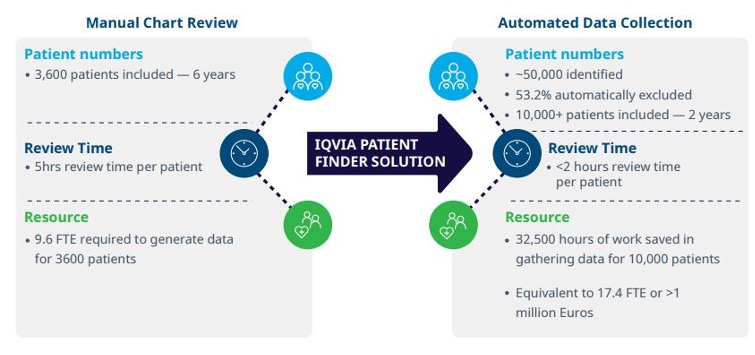
Figure 1: IQVIA, Navigating Treatment Outcomes, 2023
THE OUTCOME
IMPACT ON HEALTHCARE
Provide faster, more effective and continuous feedback to healthcare providers about their care delivery and its impact on the treatment of patients.
WIDER INSIGHTS
As well as gaining rapid, accurate insights into clinical outcomes and complications, the cost-effectiveness of treatments can be determined for the cohort.
ACCURACY ASSURANCE
Greater assurance about collecting an accurate, error-free and complete set of data in the cohort database was obtained by:
• A direct download from the Electronic Health Record (EHR) instead of manually retyping data
• Automatically unlocking all relevant data fields, which often does not happen during manual searches
HEALTHCARE COSTS
As well as gaining rapid, accurate insights into clinical outcomes and complications, the cost-effectiveness of treatments can be determined for the cohort.
TIME-SAVING
Not only is this way of searching faster than manual searching, it is less error-prone, more data can be found and the data provided is of a higher quality.
THE NUMBERS
• A registry of 10,000+ patients generated in less than 2 years
• Reduction of patients that needed to be screened for inclusion by 53.2%
• Completeness and accuracy of automated data extraction 92.3% or higher
• Identification, validation and completion of extracted data in 105 minutes per patient with patient finding technology, versus 300 minutes during the manual process (65% time saved)
• 32,500 hours of work saved, corresponding to 17.4 FTE and 1,023,333 euros saved
THE USER OUTCOME
“By using this software, we can find patients and collect their data much faster than is possible with manual data collection. This allows us to give the information back to healthcare professionals and society faster, to improve patient healthcare.” — Principal Investigator
“Before using this software, it took five hours per patient to manually transfer all the data into this database. By automating data collection, huge time savings were made, it now takes less than two hours per patient.” — Principal Investigator

For a more detailed examination of the benefits of this technology, IQVIA has published a white paper, “Finding All the Needles in the Haystack;’ Technology-Enabled Patient Identification for Clinical Trials”.
Download the white paper here.



