Takeaways from the Global Site Solutions Summit

The 2023 Global Site Solutions Summit was one to remember, hailing our biggest turnout yet. More than 1,300 industry leaders joined us to share resources, knowledge and best practices while collaborating to improve the ever-changing world of clinical research.
With more than 180 speakers across 12 plenary sessions, 40 breakouts and 8 therapeutic roundtables, many insights were shared on topics such as finances, inclusion, recruitment, staffing, business development and more.
Site Landscape
A new iteration of the annual Site Landscape presentation kicked off the Summit. Throughout the year, we’ve collected responses in micro-landscape surveys covering diversity, technology, and the site workforce. At this year’s Global Summit, we highlighted the most critical points industry stakeholders need to know.
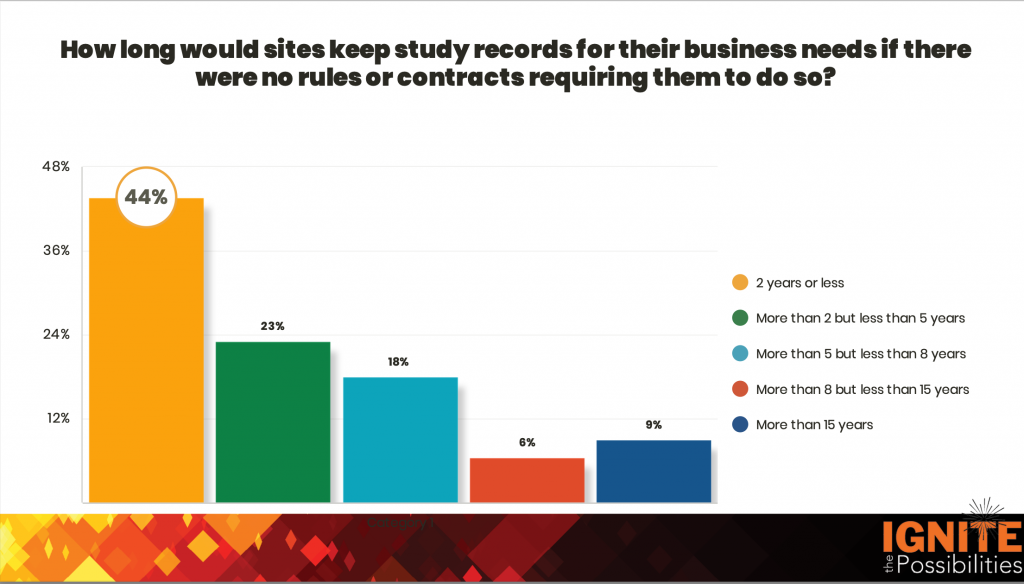
Record Retention
New for 2023, experiences with record retention and storage were requested. What we’ve learned is that the nuances of voluntary record retention beyond a site’s regulatory retention period are becoming increasingly complicated.
While some sites feel willing and capable of being long-term record storage vendors for the sponsor’s business needs, most don’t. Sites shared that they don’t want to be pushed into learning this new business. From these sentiments, it’s clear that sponsor support for alternate accommodations is needed. The most sensible option is for sponsors to use their own record storage vendors and remove the burden from the site.
Accounts Receivable
The site industry’s accounts receivable days are out of the norm for healthcare and a cause for concern. Although some sites may be partially responsible due to late delivery of deliverables, it is also heavily caused by poor payment terms in CTAs (i.e., quarterly payments and holdbacks). Even with 100% monthly payment terms, sites often see the sponsors/CROs not adhering to that schedule. There are things that are outside of our control, whether we work at a site, sponsor, or CRO, but it’s important to proactively communicate regularly and set the right expectations.
Payment Terms
The industry is making progress on getting back to monthly payments without holdbacks again. However, it is unfortunate that some sponsors are still initiating sub-optimal payment terms and switching to monthly payments without holdbacks only for the sites that ask for them. Having the cash flow necessary for optimal study conduct should not be subject only to good negotiating on behalf of the sites – it should be a standard issue in budgets.
Study delays and cancellations are much higher now than they have been previously. To account for this, we are hearing budgets and contracts allocate study delay fees or cancellation fees to keep the sites afloat during that time. Having a contingency or “catch-all” budget is also gaining more traction to cover unexpected expenses that arise for a study.
If you are involved in budgets at the site level and have not seen the SCRS Invoiceables Toolkit, this tool is available to help you calculate and negotiate budgets that better cover all your site’s costs.
Workforce
Workforce issues are still challenging sites, and several factors are at play. The most pressing challenge is the sites’ inability to compete with hyper-rising salary demands. Workforce development programs are needed for the industry as a whole, and some organizations have already begun offering scholarship and internship programs.
Additionally, due to not having enough staff members, sites are having to be more selective in the studies they undertake and the sponsors/CROs they work with. Sponsors and CROs can also help sites understand upcoming trial pipelines so they can plan better for staffing needs.
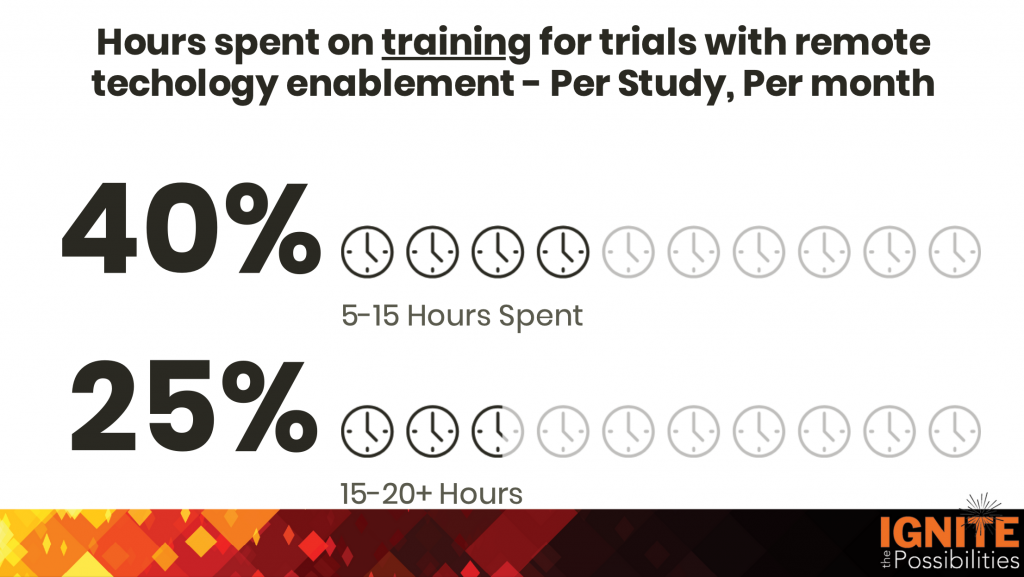
Technology & Training
As we’ve become much more familiar and comfortable with DCTs, trials with decentralized components are now the norm. Now, we’re exploring the potential of AI. Many sites and industry partners have seen how AI can be a powerful tool for streamlining and enhancing our operations. Its potential to transform or automate processes, such as streamlining recruitment or feasibility questionnaires, is truly transforming the way we conduct research.
As the use of technology in trials grows, so do training requirements. Unanimously, SCRS calls for industry partners to eliminate anything that is unnecessary, redundant or “check the box” training and shift to a more risk-based approach. Provide options. Not every site or protocol is one-size-fits-all.
Patient Perspectives
As always, SCRS aims to help you reinvigorate your “why” by featuring incredible patient stories. Zahara Weeks shared her moving journey as a patient and trial participant with sickle cell anemia. Zahara and her mom, Kesha, walked us through their journey of finding a clinical trial and how they are working to expand trial opportunities for individuals with sickle cell.
At only 14 years old, Zahara, with the help of her parents, has organized several donation drives for children with various chronic diseases and disorders, called “Marley’s Mission to Give.” In spite of the many ups and downs that life with sickle cell brings, Zahara is determined to live her best life and believes that faith and the support of her family and medical team will allow her to accomplish anything she dreams. You can learn more about Zahara’s story in her book, Born A Warrior.
SCRS is also proud to partner with Oliver Patch Project (OPP) to empower pediatric treatment participants to find their inner warriors as they endure life-changing milestone events. Brian Burkhart, who also spoke at the 2023 Global Oncology Summit, shared more about his organization’s mission to create a free, engaging platform where children with childhood cancers and their families can connect through a series of unique patches. At the Summit, Brian distributed an Ignite the Possibilities patch for Global attendees to join the mission. Learn more about Oliver Patch Project and consider making a donation.

Brian Burkhart, Oliver Patch Project 
Zahara and Kesha Weeks
Engaging Underrepresented Populations
Diversity, equity, and inclusion was also a critical topic. Speakers shed light on comprehensive approaches to developing diversity plans, offering valuable insights into sponsor expectations for sites. We heard firsthand how sponsors prioritize and implement diversity plans and the pivotal role that sites can play in driving these initiatives forward.
Ensuring diverse representation in trials is a priority for all, and sites are willing to help sponsors meet their emerging DEI goals, but not at the sacrifice of their core business. Many sites have made significant investments in diversity planning at their own expense, but not all sites are able to do this. To be successful with ongoing community engagement efforts, sites need non-study-specific resources and funding.
Sponsors and CROs have reiterated that they are willing and able to help sites with DEI-focused initiatives, but that support is first dependent on the site understanding their needs and appropriately asking for them. Sponsors and CROs can also empower staff that negotiate contracts and budgets to say “yes” without the need for multiple levels of escalation, and to do so more quickly. Developing diversity action plans can be extremely useful in providing justification for community engagement-related funds.
Celebrating Industry Innovation & Collaboration
Honoring the achievements of sites and industry partners truly strengthens our work. At the annual Eagle Award Gala & Awards Dinner, sponsored by Advarra, we recognized several organizations that have made significant impact and progress on clinical research partnerships and trial modernization:
- Eagle Award: Merck as the sponsor recipient, and Parexel as the CRO recipient
- Site Patient Recruitment Innovation Award (SPRIA): Cedar Health Research
- Site Tank: AlzWell
- Christine K. Pierre Site Impact Award: Fabian Sandoval, MD, CEO of Emerson Clinical Research Institute
- Excellence in Patient Centricity Award: Velocity Clinical Research
- Global Site Excellence in Diversity Award: Ascension St. John Clinical Research Institute
- Summit Standout Awards: Best Exhibit Design: Digital Auxilius; Warmest Smiles: Transformative Pharmaceutical Solutions (TPS); Best Aim: Helios Clinical Research; Summit Spirit Award: Lauren Stockwell from Clinical Research Fastrack
Never Stop Learning
At the Summit, there are endless opportunities to hear different perspectives, understand new technology options, and share proven strategies to streamline operations.
Now is the time to take lessons learned from the Summit back to your organizations. Share with your team and brainstorm how you can take action. If it feels like too much, focus on addressing one thing at a time. Let’s build bridges to better collaborate and #IgniteThePossibilities in clinical research.



