Site Solutions Summit Reflections

In January 2022, the SCRS team was faced with yet another pandemic dilemma. To avoid hosting an event in a surge of Omicron COVID-19 cases, we decided to move the Global Oncology Summit to May. We considered how we could expand the Summit in those four months, and the answer was clear. The SCRS Diversity Awareness Program has been growing substantially, so it was only natural that a Summit focused on diversity in clinical trials would be the most impactful. From that idea, the Diversity Site Solutions Summit became reality.
Jerome Adams, MD, MPH, former U.S. Surgeon General and Executive Director of Health Equity Initiatives at Purdue University, started the Diversity Summit by highlighting the success of Operation Warp Speed, which accelerated the development and distribution of COVID-19 vaccines and diagnostics in record time as one of the most diverse clinical trials to date.
Dr. Adams also shared a quote from Martin Luther King Jr. “Of all of the forms of inequity, injustice in health care is the most shocking and inhumane.” King said those words more than 50 years ago, and there is still much to do. That’s why SCRS wanted to host this event. Speakers representing sites, patient advocacy groups, Sponsors, CROs and solution providers discussed patient perspectives on diversity, how Sponsors and CROs can support site diversity goals, lessons and challenges with diverse patient engagement, site diversity training and much more.

A major takeaway from the Diversity Summit suggested that initiatives to engage underrepresented populations need to be sincere – not merely a reaction, checklist or industry fad. This involves ensuring sites and studies have appropriate budgets to support community outreach, participant transportation, and multi-language support or translation services, for example. Many sites expressed that Sponsors they have worked with don’t offer outreach funds to better engage with these underrepresented communities, which is something that needs to be addressed if we are to move the needle in the right direction. Inclusion should be a driving principle in clinical trial design, not just a quota. As Seneca Harrison, Chief Diversity Officer at hyperCORE International and CEO of Quality Clinical Research said, “Sponsors advocate that they want diversity, but there is no action. Don’t talk about it, be about it.”

Additionally, awareness of how to engage diverse populations is still a challenge. Simply putting research sites in a local community is not the secret to improving enrollment. We need to work with locally engaged, passionate and diverse community members and site staff to help attract more diverse participants and build trust. Explore multiple avenues for community outreach. Have an open house, lunch and learn, or host community events. Work with local physicians to increase awareness and improve accessibility to clinical trials. Patient advocate Sheila Johnson commented, “Go into communities and talk about clinical trials. Show up with love and concern. Know your patients. Be culturally sensitive. Don’t invite patients to the table after the table is already formed.”
Site diversity training should be at the top of every Sponsor’s to-do list. That training should discuss the meaning of diversity, what it means to enroll diverse patients, and how targets can be set. Sites not only need to recruit diverse populations but also need to know how to retain them. Train sites on how to actually engage and build relationships with underrepresented communities.
Changing the dialogue surrounding diversity in trials starts with education, access and trust. The SCRS Diversity Site Assessment Tool, also known as the DSAT, encourages sites to analyze their knowledge and capability of meeting diverse enrollment goals. Several Sponsors are already encouraging the DSAT to be completed as a prerequisite for sites before study opportunities are provided.
Sandy Amaro, Head of Clinical Trial Diversity at Pfizer commented, “It was such an honor to attend the first Diversity Summit. To be surrounded with so many passionate people gave me more energy and drive to move this work forward at a faster pace. I look forward to what we can achieve together over the next 12 months. We need to go into the second Diversity Summit talking about actions taken and impacts made!”
Nearly 400 sites, Sponsors, CROs, solution providers, patient advocacy groups and students joined us at the inaugural Diversity Summit. Thank you to Total Diversity Clinical Trial Management and Parexel for generously providing registration scholarships for research sites, patient advocacy groups and students to attend. It’s clear from the conversations at the Summit that there is still much work to do, but we are headed in the right direction. Coming together to collaborate, share ideas and best practices, and discuss solutions for how to move forward together made an impact. SCRS encourages everyone that joined us to share what you learned with your organization and network. We look forward to growing the impact of the Diversity Site Solutions Summit as we extend the conference to one-and-a-half days. Join us next year in Austin, Texas March 30-31, 2023!
SCRS Oncology Community Reunited
This year, we finally reunited with our oncology community again in person after holding the event virtually in 2021. Scheduled immediately after the Diversity Summit, the Oncology Summit saw record registration with nearly 400 attendees. Voices from sites, Sponsors, CROs, solution providers and patients discussed topics such as site budgets, technology, workforce challenges, and diversity specific to oncology clinical research.
A favorite session for Summit attendees is always the Site Landscape Survey, and SCRS collected oncology-specific data to represent challenges and insights facing cancer research today. One of the biggest obstacles the SCRS Site Landscape Survey explored is site staffing. Survey results showed that 63% of oncology research sites have encountered significant challenges maintaining their current workforce over the last 18 months. The role with biggest reductions and hiring challenges is unsurprisingly site coordinators, due to a lack of experienced individuals in the job pool, sites’ lack of financial ability to compensate employees at current salary rates, and employees leaving for other opportunities.
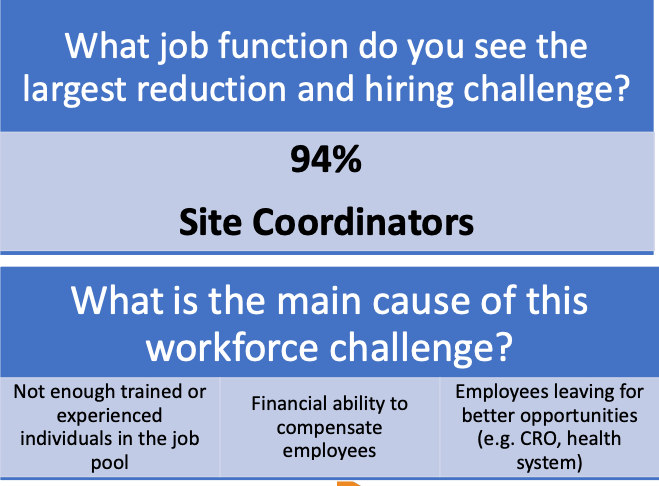
What can Sponsors and CROs do to help alleviate this challenge? Sites indicated providing adequate budgets to support the hiring of needed staff is the most critical need. Additionally, providing an active flow of trial opportunities, support for training new research personnel and support for hiring and applicant marketing would help sites address workforce challenges.
Decentralized trials continue to be a focus of discussion for the industry, yet 63% of oncology sites surveyed said they were not approached to conduct a decentralized oncology trial. 43% of oncology sites that were approached for a decentralized trial declined to participate. Why? 50% of the oncology sites said they are in a “wait-and-see” approach for decentralized trials. By contrast, 66% of sites in other therapeutic areas were approached to conduct a decentralized trial. Clearly there is a need for oncology-specific technologies, opportunities, and education.
Increasing diversity in oncology trials was also a hot topic of the Summit. Deena Bernstein, VP of Customer Success at Datacubed Health, led a session on strategies to increase minority enrollment in oncology trials. “We discussed how cancer trials have a high level of complexity. Adding another challenge to them is the enrollment of diverse and minority populations,” said Bernstein. “With diverse representation in oncology trials, we know that we will have access to more accurate, robust outcomes to represent more cancer patients. Our panel and audience talked and shared insights about how education, community-based healthcare providers on the front line, addressing health literacy, and including patient optionality are the things we all need to focus on to achieve the goal of reaching more diverse people to participate in oncology trials.”
Underserved populations can benefit from increased access to novel drugs in clinical trials, rather than waiting for drugs to become FDA-approved treatments, yet they aren’t participating. Bernstein added that the session attendees discussed how proper education about the trial can help move the needle. Also, many patients are unaware that placebos aren’t used in cancer trials and treatment would never be held back. Involving the patient’s family throughout the trial is important to ensure everyone understands and trusts the process. Involvement from primary care providers (PCPs) and communication between PCPs and oncologists are important for building trust and continuity of care. Lastly, introducing a clinical trial in a comfortable setting by a Nurse Navigator (a liaison between the patient and clinical care staff) can help navigate the patient through the clinical trial and treatment process and help patients make informed decisions.
Kim Kundert, SVP of Site Development Services at Total Diversity Clinical Trial Management also participated in a diversity and inclusion-focused session, commenting that the enthusiasm of the audience – which included all industry stakeholders – was memorable. “As a presenter, you always worry about lack of audience participation or questions when you ask them. That was not the case in this session. There was a sense of collaboration which isn’t always commonplace in the clinical trial industry.”
A recurring theme throughout every SCRS event is that Sponsors are encouraging sites to ask for what they want and need. “Everyone knows that in order to move the needle on diversity in oncology trials and clinical trials in general, it will take all of us working together,” said Kundert.
Another Summit attendee added, “I absolutely loved the stakeholder comradery bringing deep insights on how to operate oncology trials. It was refreshing to see more consideration around the patient perspective in study design, reimbursements for sites and participants, and inclusivity to remove burdens for all.”

At the end of the Summit, Peter Fredette, Oncology Strategic Site Solutions Director at IQVIA, was announced as the new SCRS Oncology Board Chair. After the Summit, Peter shared goals for his new role with SCRS as well as his favorite moments from the event on the SCRS Talks podcast. Registration for the 2023 Global Oncology Summit from March 31-April 1, 2023 in Austin, Texas is open now.
Highlights from Australia-New Zealand
It’s hard to believe it had been more than two years since the Australia-New Zealand community came together in person. Once again, SCRS was proud to see registration for this Summit grow to record levels. We always do our best to ensure an equal representation of sites and industry at our events, and the ANZ Summit was evenly split 50/50!
SCRS VP of Site Engagement, Jimmy Bechtel, kicked off the Summit with SCRS Honorary President David Vulcano and Tam Nguyen, Associate Professor and Deputy Director of Research at St. Vincent’s Hospital Melbourne with the Australia-New Zealand Site Landscape Survey insights. Other plenary sessions included the importance of site collaboration, looking at how the clinical research profession has changed and the trial compensation process in Australia.
All sessions received high marks, but the “Exploration of Clinical Research as a Profession” plenary that shared a patient’s perspective made a notable impact. Discussing challenges the patient went through after an adverse reaction to the treatment in the trial, it was a call to action for the industry to reform the trial adverse event reimbursement process. Cheryl Ann Hawkins, Chief Operating Officer at Emeritus Research added, “This session had massive engagement and interest, and I have learned that patients need to have access to much improved legal support in Australia if they were to suffer serious side effects from a clinical trial. Looking forward to part two next year!”
Attendees also enjoyed breakout sessions as unique opportunities to network while discussing the CT:IQ InFORMED Project, study start-up, site audits and managing expectations with Sponsors and CROs.
Vanessa Irvine from Gallipoli Medical Research Foundation reflected on the Summit, saying “This was my first SCRS conference experience, and I will be going back for sure! It was great to meet others in the industry, and really feel like it was a collaboration. I went back to my site and couldn’t stop talking to my staff about all I had heard.” Dates for the 2023 Australia-New Zealand Site Solutions Summit will be announced soon. With the growth of the Summit, we are exploring new venues which may be the new home for the July 2023 Summit in Melbourne, Australia. If you are an SCRS member or Global Impact Partner, contact info@myscrs.org for a special registration code for all SCRS Summits.