April 2019
Welcome to the April issue of InFocus, where we provide insights and solutions to help sites and other stakeholders ensure site sustainability.
SCRS Current
SCRS is committed to continually advocate on behalf of our member sites to ensure they remain sustainable and have access to the tools needed to grow their business.
SCRS members and Summit attendees have shared concern around attempts to create a 100% virtual, or “site-less”, clinical trial model, which would directly impact the availability of trials to sites.
SCRS believes that the value clinical research sites bring to the industry is not replaceable. The site-to-patient relationship is critical to our industry’s success.
The future of clinical research must include site-centric technology. Together, the site community can provide the leadership needed to improve the patient experience through the utilization of forward-thinking technology solutions.
In dedication to ensuring long-term site sustainability, the SCRS team is in the process of creating a digital innovation initiative that will explore the impact of clinical trial technologies on research, and inform members on the importance of understanding and utilizing digital capabilities.
An objective of this initiative is to provide SCRS member sites with digital intelligence and a competitive advantage in the marketplace.
This is an important initiative, and SCRS has already received enthusiastic support to help build a comprehensive program to offer to our members. We look forward to sharing more details with you soon. If you have any questions in the meantime, please reach out to us.
MetricsThatMatter
Improving the Patient’s Clinical Trial Awareness and Experience
Every involved party is integral to a clinical trial’s success, but none more than the patient.
Trials come to a halt without willing participation from qualified patients who have the ability to meet the trial’s needs. Yet it is only in the last several years that we have we begun to look at the patient’s experience to inform clinical trial development. Failure to engage patients has led to an incomplete understanding of clinical trials and lacking awareness of the benefits of participation.
According to CISCRP, 75% of the general public is willing to participate in a clinical trial, yet only 30% have a basic understanding of what clinical research is. These data demonstrate that potential patients want to engage in research – they just don’t know where to turn. Only 21% of the North American public is confident in their ability to find an appropriate research study. Those located in South America indicated the highest confidence level of finding an appropriate trial (27%), while those in the Asian Pacific region indicated the lowest confidence level (10%).
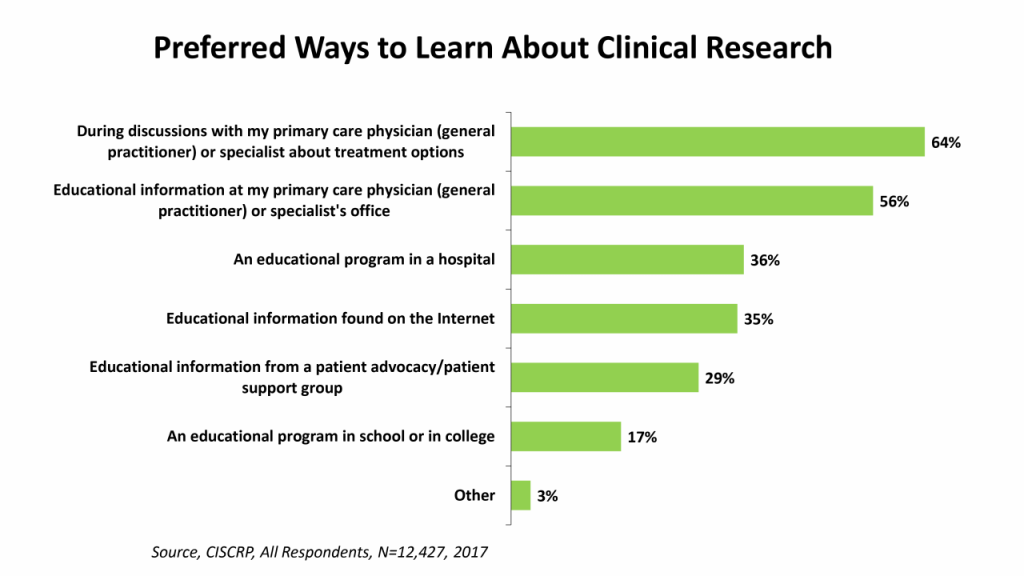 Who can potential patients turn to for the education they need to inform their decision to participate in clinical research? The majority – 64% – prefer to receive information about clinical research as a treatment option from their primary care physician or specialist, and 56% want access to educational information at their physician’s office. However, only 19% reported that this is where educational information was received.
Who can potential patients turn to for the education they need to inform their decision to participate in clinical research? The majority – 64% – prefer to receive information about clinical research as a treatment option from their primary care physician or specialist, and 56% want access to educational information at their physician’s office. However, only 19% reported that this is where educational information was received.
Despite the prevalence of general online information-seeking, only 35% of those surveyed indicated a desire to find this educational information on the internet.
The desire for information is there, but the knowledge isn’t, which has created an environment where only 3% of those interested in participating in a clinical trial are actually able to.
Clearly, this is not a result of a lack of desire or need. How do we change this reality?
“An investment in knowledge pays the best interest.” – Benjamin Franklin
Education for potential patients is critically needed, but it is equally important to educate their physicians. “What we are lacking is the proper reach to eligible patients and their providers,” said SCRS’ senior project manager, Jimmy Bechtel.
While some care providers either talk to their patients about clinical trials as a care option or provide information on clinical trials in their waiting rooms, many providers do not have the knowledge necessary to provide this information to their patients because they don’t know where to direct them. A patient in the audience at the April 17 AWARE for All: Clinical Research Education Day in Baltimore, MD, shared, “I tried to get this information from my doctor’s office, but they didn’t know where to send me.” It is imperative not only that clinical research as a care option exists, but that physicians know how to connect their patients with clinical trials. Though it can be difficult to navigate, physicians can direct patients to clinicaltrials.gov or help them search the website to find a clinical trial. Many local hospitals, sites, sponsor and CRO companies, and organizations like CISCRP provide a list of available clinical trials as well. Knowing where to find this information will empower physicians to offer access to clinical trials as a treatment option to their patients, while simultaneously informing patients about what clinical research is.
In addition to providing education across the entire spectrum, we need to identify ways to make the experience of clinical trial participation more positive for patients. Doing so will encourage the spread of clinical trial awareness via word-of-mouth. Many organizations are working toward this end, implementing innovative approaches to improving the patient experience such as including patients in protocol development and bringing patients to investigator meetings to talk about their experience as a clinical trial participant.
With increasing education and awareness among patients and practitioners as our overarching goals, we will empower the industry to become more patient-informed and patient-centric.
Reference: CISCRP Charts and Statistics. Accessed 4/16/2019 from: https://www.ciscrp.org/education-center/charts-and-statistics/
Successful Techniques for Accelerating Recruitment (STAR)
Utilizing Connection as a Recruitment Tool
 Research has shown that “strong social connection strengthens the immune system, aids recovery from disease, and leads to a 50% increase in life expectancy.”1 It is only logical that this same tool could be utilized to boost recruitment at your site.
Research has shown that “strong social connection strengthens the immune system, aids recovery from disease, and leads to a 50% increase in life expectancy.”1 It is only logical that this same tool could be utilized to boost recruitment at your site.
As clinical trials increasingly move toward incorporating virtual visits and various technologies, it is even more important that physicians continue to prioritize quality relationships with their patients. People invariably trust people, and while the incorporation of telemedicine is undeniably needed, so, too, is the connection between doctor and patient.
As your site moves toward adopting telemedicine practices, are you continuing to prioritize quality relationships between your doctors and their patients? Are you keeping the human element of clinical research in mind? This can be as simple as personal follow-ups after a remote visit, allowing 5 more minutes to address any concerns or questions after an in-person visit, or something as simple as eye contact and not rushing through the visit.
Quality patient engagement is the tool that will allow your site to succeed in the telemedicine space, and is one of the best business practices your site can utilize to boost recruitment and retention success.
Reference: The Changing Face of Clinical Research. SCRS. March 13, 2019. Accessed April 19, 2019 from: https://cloud.3dissue.com/180561/181052/211361/InSiteSpring2019/index.html?page=10
TransCelerateUpdate
Improving the Site and Sponsor Relationship through Clinical Trial Protocol Design
“Today’s clinical trials are designed for unicorns” is a phrase often communicated by those who are closest to executing clinical trials. Many site investigators, study coordinators and study participants believe that today’s protocol designs have unrealistically narrow inclusion and exclusion profiles, posing complex recruitment challenges for study sites and making finding the “right” study participant quite difficult. Additionally, protocols continue to be designed with increasing complexity, whereby the understanding by study sites and participants is, at best, onerous. Collectively, these pain points make the interpretation and execution of clinical trials burdensome and exhausting for investigator sites and their study participants.
TransCelerate has been working diligently to create solutions that will improve protocol designs with the aim of improving their understanding by study sites, reducing the recruitment burden, and incorporating the patient perspective early in design. This article is the first in a multi-part series that addresses steps underway to positively impact the site and sponsor relationship of the future.
Pain point: Protocol designs are not realistic
To improve upon the common problem in which sponsors are designing protocols for “unicorn” study participants, TransCelerate’s Placebo and/or Standard of Care (PSoC) initiative is one whereby the aim of the initiative is to improve protocol designs and reduce their complexity. Since the initiative’s inception, participating Member Companies have shared over 130 de-identified studies involving study participant level data from over 84,000 patients in 19 different disease areas. The benefits to pooling and sharing these important data include:
- Inclusion and exclusion optimization by better screening selectivity on the number of study participants required
- Control arm substitution, yielding a reduction in the size of the PSoC control arm in clinical trials
- Improved precision in sample size calculation
- Better understanding of geographical relationships among places and people and the differences in study participation
- Better understanding of how diseases develop
TransCelerate’s PSoC Initiative has created several manuscripts that provide a view into the different use cases defined and relevant, acceptable statistical methods and approaches to take when utilizing PSoC data to inform protocol design.
Pain point: Improving understandability of the complex protocol
Complex protocol designs are challenging. Such protocols read like legal documents and often include information that is not related to the successful execution of the trial. As a result, there is an increasing burden on the sites to try to understand and follow these complex instructions.
The Common Protocol Toolkit (CPT) initiative, with stakeholder input from site investigators, study coordinators, members of IRBs, IECs, the TransCelerate CRO Forum, and multiple regulatory authorities, created a well-adopted, common clinical trial protocol template that contains a common structure harmonized with FDA/NIH and model language to improve protocol understanding, review and approvals, accuracy in data recordation and, ultimately, the speed of study start-up. Additionally, the CPT includes proposed model text and regulator-accepted endpoint definitions that may be used across protocols with minimal to no editing needed. The CPT is available to anyone within the eco-system as a MSWord document and as a Technically-Enabled Edition (TEE) whereby add-ins have been embedded to facilitate the authoring, population, and finalization of the document.
By the end of 2019, TransCelerate will be releasing the next version of the CPT with additional content re-use capabilities into two additional common templates developed within TransCelerate. These include the Common Statistical Analysis Plan (SAP) Template as well as the Common Clinical Study Report (CSR) Template; both providing a common layout within each respective document. Collectively, these three documents provide an important foundational step in enabling the automation of end-to-end data flow through content re-use and downstream, process automation.
Pain point: Protocol designs lack the voice of the patient
The Patient Protocol Engagement Template (P-PET) is designed to help sponsors design clinical trials with participant input. The P-PET provides tools and resources to use in engaging patient advisors during protocol development with the goal to improve participant experience and reduce participant burden during the study. The goal is to increase the patient perspective early in design to reduce patient burden (i.e. scheduling visits, invasive procedures), leading to better recruitment and retention of study participants.
For example, in the development of this tool, TransCelerate’s Member Companies discovered that eight-hour visit schedules and test procedures are tiresome for both sites and participants. Participants would frequently request that treatment be split into two four-hour sessions over two days, rather than one day-long session.
The benefits in including participant feedback into a protocol design can be felt both near-term and long-term, including:
- Improve the participant’s experience in clinical studies
- Improve the number of participants joining in clinical studies
- Reduce protocol amendments and study dropout rates
- Speed up the delivery of medicines to market
- Support development for more “fit for patient” drugs
The P-PET is targeted towards the beginning of a study including the stages of target product profile, the clinical development plan, the protocol concept, and protocol optimization. It’s comprised of three parts to create one holistic approach:
- User Guide: Supports sponsors in understanding the value of implementing the P-PET in the development of clinical studies; having meaningful discussions with patient partners during the development of a clinical study protocol; socializing the patient partnering process with stakeholders to achieve greater support as needed; and example case studies
- Resource Guide: Examples of visual aids are provided to facilitate clear communication of study design and protocol-related concepts to patientsTemplates:
- Patient Advisory Board Pre-Read
- Patient Advisory Board Sample Presentation
- Satisfaction Survey – Patient Advisor
- Satisfaction Survey – Study Team
- Thank You Note
- Patient Advisor Report
- Study Team Report
- Satisfaction Survey: Support in capturing lessons learned
The P-PET will be free to all users and will be accessible for download on TransCelerate’s website in early Q3 2019.
Check back here in a future issue for the next article in our “Creating the Site and Sponsor Relationship of the Future” series. Subsequent articles will cover how to improve access to trials and how to improve participation in trials.
UpcomingWebinars
JOIN US AT AN UPCOMING SUMMIT
AboutSCRS
Founded in 2012, SCRS is a global trade organization that unifies the voice of the clinical research site community to create greater site sustainability. Representing over 9,500 sites in 47 countries, SCRS membership provides sites with a community dedicated to advocacy, education, connectivity and mentorship. SCRS is an influential voice for sites and an active partner in industry-wide initiatives and dialogues focused on improving the clinical research enterprise. Our Voice. Our Community. Your Success. Join the community.







