Expanding Collaboration in Clinical Trials through First-of-Its-Kind Site-Sponsor Shared SSU Milestone Tracker

Clinical research continues to evolve toward greater connectivity and efficiency, yet study startup remains one of the most complex and time-consuming stages of a trial. Disjointed systems and communication gaps between sponsors, CROs, and research sites often lead to avoidable delays. To address these challenges, Advarra has expanded its Study Collaboration solution to better support sponsor and CRO study teams in their startup collaboration with sites, with a site-facing milestone tracker designed to streamline coordination and strengthen alignment across all study stakeholders.
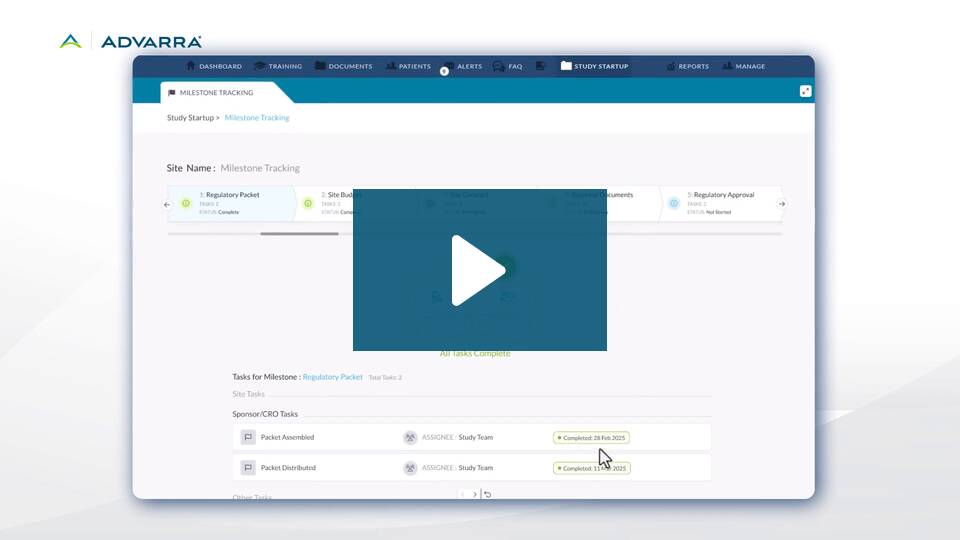
The shared milestone tracker gives research sites real-time visibility into activation progress alongside sponsors and CROs. This provides a unified workflow where all team members can manage startup responsibilities within the same system.
Improving Transparency Across Study Stakeholders
Advarra’s Study Collaboration solution connects study teams and sites to activate and plan for enrollment via document exchange, study training, centralized communication, and enrollment planning support. The latest feature builds on that foundation by giving sites their own real-time view into activation progress, in addition to existing milestone tracking for sponsor and CRO study teams.
The site-facing milestone tracking capabilities empower site staff to manage their responsibilities within the same workflow used by sponsors and CROs. This site-facing milestone tracker enables sites to follow their own internal processes while automatically updating shared milestones. The result: a dynamic, synchronized system that replaces the old cycle of spreadsheets, emails, and recurring status calls.
“Too often, study startup is hampered by fragmented systems and poor communication,” said Ashley Davidson, VP, product lead of sponsor tech strategy at Advarra. “Advarra’s latest release solves this challenge with the market’s only site-facing milestone tracker aligned to the sponsor and CRO view, bringing all research team members into a single, coordinated workflow.”
Collaboration Built with Sites, for Sites
Developed in close partnership with Advarra’s extensive network of research sites, the new functionality reflects direct input from end users to ensure it aligns with real-world workflows.
“It’s truly exciting to see a company that actively incorporates end-user site feedback to build purpose-driven solutions,” said Nicholas Focil, CEO of FOMAT Medical Research.
By co-developing with the sites that use the technology every day, Advarra ensures its tools reflect how research is actually conducted, supporting greater usability and adoption across study teams.
A Clearer View into Study Activation
Sites adopting the platform report that it creates a more transparent and predictable startup process.
“Study startup has long felt like a black box,” said Nancy Cleverley, SVP of operations management at AMR Clinical. “With Advarra’s Study Collaboration solution, we can open that box and give everyone the same view of clinical trial activation. With this new level of transparency, collaboration becomes easier, timelines become clearer, and we can focus less on managing communication and more on activating studies and enrolling patients.”
The site-facing milestone tracker builds on Advarra’s earlier integration of Study Collaboration with its Center for IRB Intelligence (CIRBI®) system. Together, the two solutions bridge regulatory and operational workflows to accelerate study activation while maintaining compliance and data integrity.
Accelerating Study Startup Through Connection
With this release, Advarra strengthens its mission to improve research efficiency and accelerate study startup through better-connected teams. The result is a more transparent, site-centered, and collaborative model for clinical research that is built to keep trials moving forward.