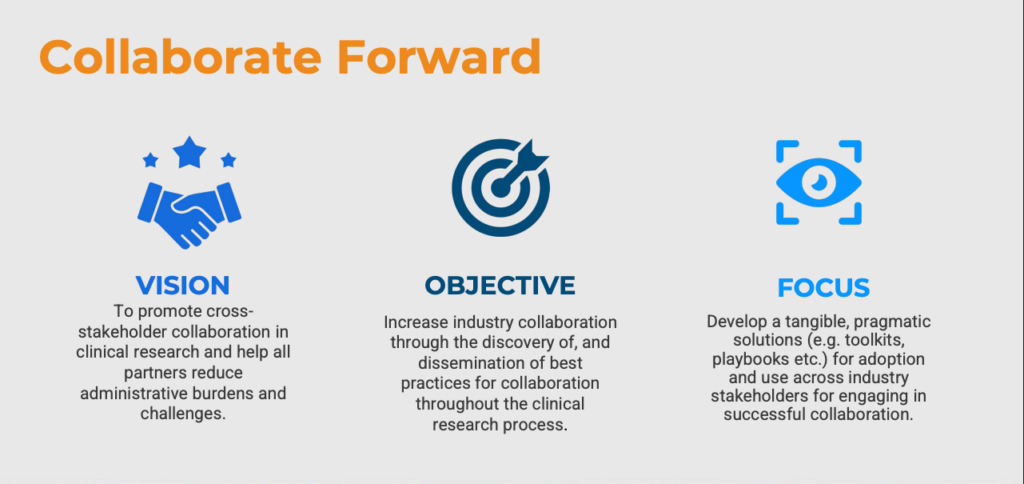
Collaborate Forward: Advancing Clinical Research Through Collaboration

Collaboration is the cornerstone of clinical research success. Yet, as the industry continues to evolve with increasing complexity and workforce turnover, maintaining effective collaboration remains a significant challenge. According to the Tufts Center for the Study of Drug Development, 70% of global research site staff stated that trials have become more challenging to manage in the last five years due to increasing complexity. Between 2008 and 2019, trial complexity drove a 39% increase in trial timelines, amplifying the need for better coordination and efficiency.
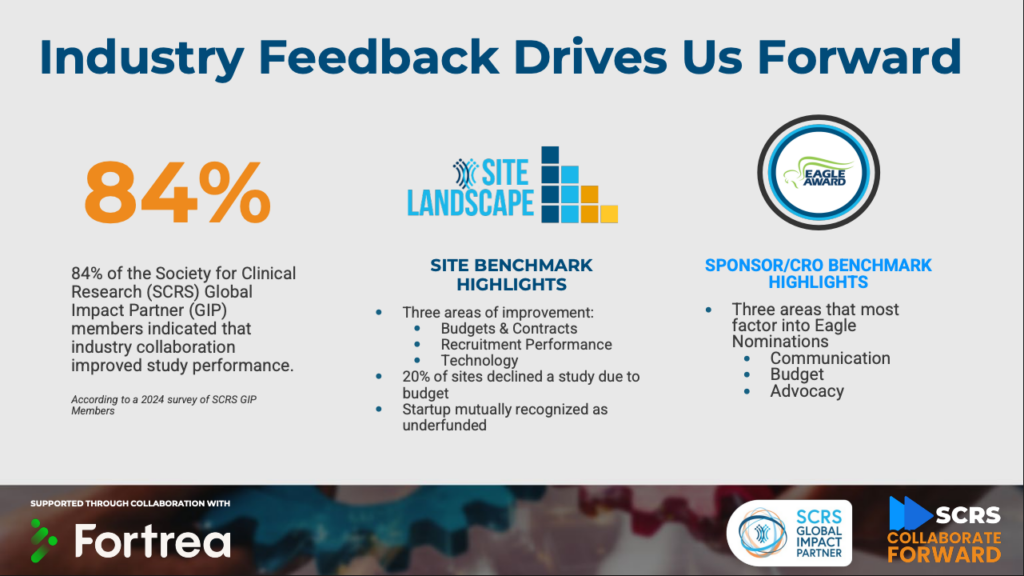
Taking a deeper look at SCRS Global Impact Partner (GIP) members, 84% indicated that industry collaboration improved study performance, and data from the annual Site Landscape Survey and Eagle Award nominations highlight communication, budgets & contracts, and recruitment performance as key areas needing improvement. Recognizing these challenges, the SCRS GIP Member Community has launched Collaborate Forward, supported by Fortrea. The initiative is designed to explore, define, and implement best practices for cross-stakeholder collaboration in clinical research.
Why It Matters
Effective collaboration is not just a theoretical advantage—it’s a competitive one. The increasing complexity of clinical trials, coupled with workforce turnover, has led to higher costs, inefficiencies, delays in study start up, and lost productivity.
“Simply put, there is a need to overcome these complexities for the sake of the patients and the industry,” said Mike Clay, Senior Vice President of Global Project Delivery at Fortrea. “We believe that collaboration with clinical research sites is key to unlocking efficiencies and productivity gains that will streamline the clinical trial process. By streamlining cooperation among sites, sponsors, CROs, and solution providers, the industry can reduce delays and improve study outcomes. As a leading CRO, we are proud to be at the forefront of this effort, ensuring that sites remain central to driving progress and fostering greater industry-wide collaboration to bring life-changing treatments to patients faster.”
Collaborate Forward seeks to provide the industry with the tools needed to turn collaboration into a strategic advantage, improving both efficiency and effectiveness in clinical trials. To tackle these challenges head-on, Collaboration Forward is transforming collaboration from an industry-wide challenge into a strategic advantage.
A Playbook for Effective Collaboration
The Collaborate Forward initiative is not just about discussion; it’s about action. Our initiative is developing a playbook that serves as a guide for successful cross-stakeholder collaboration. This playbook will be built on real-world insights, highlighting practical strategies that drive efficiency and effectiveness in clinical trials. The playbook can be used by clinical research sites, site networks, sponsor companies, CROs, and solution providers to analyze and improve their own collaborative processes, or take on new collaborative endeavors.
The playbook will be available in 2026, and encompass 11 stages of clinical research. 2025 efforts will focus on three critical phases identified by GIP members:
- Study Feasibility – Ensuring studies are designed with practical execution in mind.
- Contracts & Budget Negotiations – Streamlining agreements to minimize delays and inefficiencies.
- Patient Recruitment – Identifying strategies to improve enrollment and retention.
How We’re Doing It
Before developing collaboration solutions, we must first define the conditions necessary for success. Without the right conditions, or as we call them, “principles”, the collaboration is at risk of failure, no matter the size nor scope of the work. Bottom line: We know there are stories of collaborative success in the industry – we’ve all seen them – but what commonalities do they all share that we can learn from?
As the team examines best practice examples, they’ll focus on the principles: Project – the mindset and motivations for collaboration, People & Connection – who is involved and how information is disseminated, Process – alignment on the purposes and requirements, and Progress – how has this inspired future collaborations.
The Collaborate Forward project follows a structured approach:
- Stakeholder Interviews – Collecting first-hand accounts from industry professionals to understand real-world examples of collaborative success.
- Analysis of Data – After interviews, analyze the examples against the principles of collaboration, identify metrics, and review any lessons learned.
- Develop Summaries – Create a summary for each study phase that includes competitive advantages.
- Creating the Playbook – A tangible, pragmatic guide to drive collaborative success across clinical research stakeholders. This playbook includes all phase summaries, interviews, case studies, articles, supporting data, and recommendations.
Get Involved
We invite professionals across the clinical research industry to contribute to this effort. Whether you have a success story to share, insights into overcoming collaboration challenges, or a desire to be part of shaping the future of clinical research partnerships, we want to hear from you. Your insights could play a key role in shaping the future of collaboration in clinical research.
Learn more about our work, how to participate, or how to share your story by visiting https://www.scrsprograms.com/collaborate-forward/. For more information on Collaborate Forward, please reach out to Brian Egan or Sean Soth.
Together, we can build a more connected, efficient, and impactful clinical research ecosystem.



