A Few Clicks and Done: Automating EDC Data Entry
By Dan Braga, Vice President, Product Management, EHR and Healthcare Solutions, Medidata
Repetition—depending on the task at hand—can be a positive or negative thing. On the positive side, repetition may assist in honing skills, enabling us to become proficient in what we do, whatever that may be. On the negative side, repeating specific tasks may be time-consuming, tedious, frustrating, or stressful.
Data re-entry has been an issue in clinical trials for as long as many of us can remember. The question often asked is, why should busy professionals be forced to spend their limited time duplicating data that has been captured previously and is available from other sources, such as Electronic Health Records (EHRs), Clinical Trial Management Systems (CTMS), eSource, documents (e.g., lab values in a spreadsheet), or other systems?
According to a survey conducted by Medidata and the Society for Clinical Research Sites in 2022, 98% of sites reported that they manually re-enter data into Electronic Data Capture (EDC) systems, with nearly 70% of them stating that more than 50% of their EDC data is re-entered using existing EHR data. This is inefficient, and it must be frustrating for research coordinators who could be spending time on more valuable study tasks or with their patients.
In addition to the vast amount of time that this process takes, there is a high probability that transcription errors will be introduced, resulting in additional time and effort being spent by monitors and data managers on raising queries and by sites on resolving them—a laborious undertaking, given that the average number of queries per Phase III study was found to be 96,980 (Stokman, 2021). Also, remember that the data generated by clinical trials continues to increase yearly, adding to the growing burden.
So, what has been done to resolve the issue, and why is it taking so long to find a solution? Isn’t technology meant to reduce or remove problems like this?
Two Steps Forward and One Step Back
Issues were recognized many years ago. EDC systems have long been used to collect, clean, transfer, and process clinical data. Efforts have been made by clinical researchers and technology providers to repurpose EHR data in a scalable way. Certain attempts to address the issues have shown great promise, and many positive steps have been taken to improve efficiency and reduce errors. Still, adoption and progress have been slow, with little change until recently.
Additionally, automating EHR to EDC data transfer and integration benefits include reduced trial costs, earlier identification of safety events, and faster trial completion. With increasing complexity in clinical trials and the sheer volume of data being processed, automation reduces the burden of data entry and reduces data quality issues.
On the other hand, in the background, progress has been slowed by issues that most users will not be aware of. For a start, the industry was initially working without universal standards, which we’ll come to in a moment.
One limitation is the inability of specific EHR systems to interoperate (communicate and work together) with other EHRs. Another is the inability of data entry/transfer automation solutions to work with non-EHR data; some are limited to working with just a few selected EHRs. Variation in configuration from one EHR to another presents an additional issue.
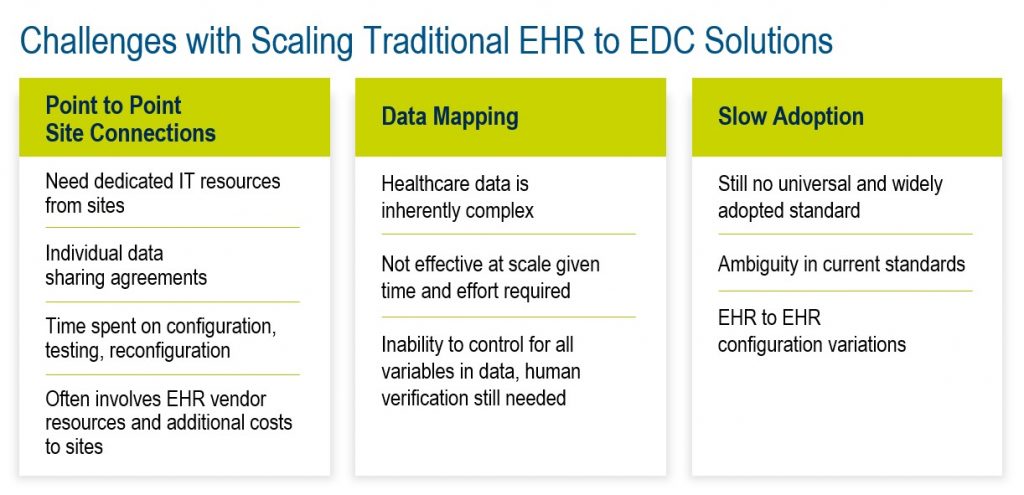
These challenges have led to longer implementation times due to complexity, higher resource requirements, and higher costs. This has meant that, while they have demonstrated promising results on single studies with a small number of sites, solutions have not proven to be easily scalable to multiple studies with large sites.
Fortunately, a couple of crucial changes took place, paving the way for creating a new system that addresses the industry’s challenges and reduces the burden of data entry and re-entry for clinical trial professionals.
The Turning Point
On December 13, 2016, the 21st Century Cures Act was signed into law in the United States, with one of its aims being to enhance interoperability and reduce regulatory burdens associated with sharing healthcare data. This was a huge step, enabling specialist service and systems providers to create an interoperable ecosystem of solutions that help research sites and end-users.
Additionally, EHR data interoperability was standardized by the Office of the National Coordinator for Health Information Technology (ONC) when they recommended using the HL7® FHIR® (Fast Healthcare Interoperability Resources) standard.
Although extracting data from EHRs and feeding them into EDC systems remains complex, these changes have provided a tailwind and a standardized approach for data exchange. Furthermore, regulatory agencies have recognized the value of EHR data in clinical research and encouraged its use in guidance and recommendations.
These changes enabled the creation of technology solutions that address industry challenges based on standards and interoperability.
Is There a Solution Now?
In 2022, a unique solution that exceeded the industry’s limited prior offerings and set a new standard was unveiled. The new system introduced interoperability beyond EHR and EDCs, expanding connectivity across multiple data sources while significantly improving the user experience.
Using an alternative approach to other systems—one that doesn’t try to automate the process fully—provides easy-to-use tools that assist the Clinical Research Coordinator (CRC) in completing EDC forms (eCRFs – electronic case report forms) much more easily and quickly. Because it doesn’t rely on costly, time-consuming, upfront mappings between EHR and EDC systems, which can change from study to study and system to system, set-up is quicker, easier, and scalable across multiple studies and sites. No automated solution has proven 100% accurate mapping between an EHR and an EDC’s specific clinical trial visit records. With the new system, the CRC always controls which data is submitted into the EDC system.
The new solution takes a multi-pronged approach, leveraging multiple ways of getting data, and taps into existing networks and interfaces, including direct connections to sites’ EHR systems and data aggregation platforms.
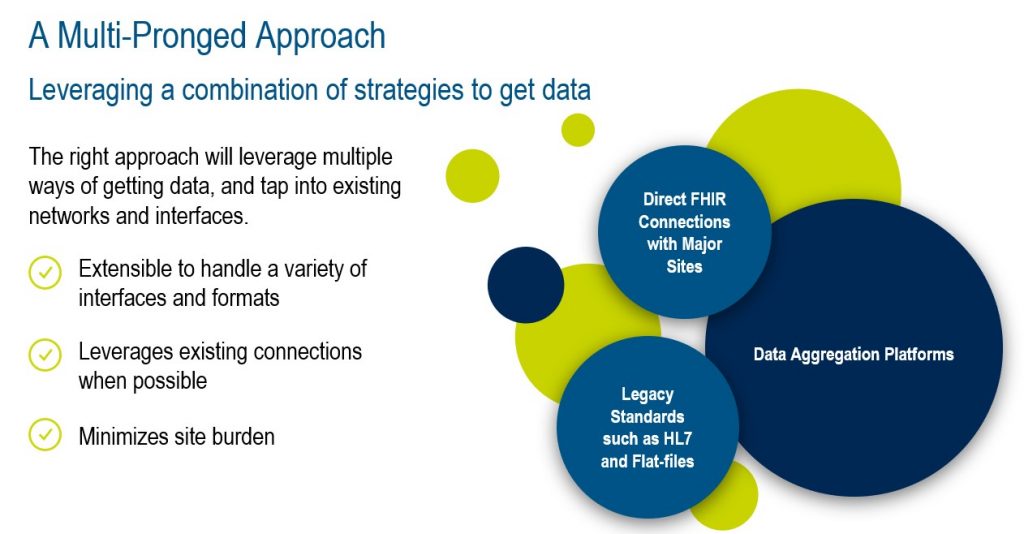
From a site user perspective, the solution is a data entry assistant that allows research coordinators to retain complete control over what is submitted into the EDC system. It supports structured and unstructured data, so it is simple to capture data from any source application or document. As it can operate with or without connection to an EHR system, it offers flexibility and adaptability to different workflows while leveraging multiple connectivity options whenever a link is necessary. With its more straightforward approach, the solution eliminates the need for an EHR to trial protocol mapping exercise, saving valuable time and effort for the research team.
Most importantly, it removes the need for a highly tedious and time-consuming data re-entry process, replacing it ‘with just a few clicks’ for the user.
Major US sites and site networks are rolling out this solution, and they’re seeing highly positive results, with site users finding it very easy to install and use; more importantly, they’re seeing significant time savings in completing EDC forms. At one site, the time usually taken to complete an EDC form, requiring 48 lab values and seven other values to be entered manually (looking up the data in the EHR or other system/document and re-entering it into the EDC form), was 22 minutes. With the new data entry assistant solution, the same form, with the same data, can be completed in just 30 seconds. That’s 44 times faster, meaning that sites can complete data entry for more patients in less time, freeing them up for more valuable clinical research and patient care activities.
An excellent white paper on this topic can be found here, and if you love podcasts, here is a link to the SCRS Talks Podcast with Jimmy Bechtel, MBA, VP Site Engagement, SCRS, and Dan Braga, Vice President, Product Management, EHR and Healthcare Solutions, Medidata.
Summary
By addressing interoperability challenges, enabling other source data to be captured quickly, and simplifying data entry, the new solution provides a user-friendly, simple solution for clinical researchers, eliminating the need to bounce between applications and making data entry and re-entry easier, faster, and more accurate.
It means fewer queries, an easier workday, and more time to focus on critical tasks and patients. The result? Happy sites, CROs, Sponsors, and patients. And all this is achieved “with just a few clicks.”