Accelerating Study Startup

Systems that are readily accessible at the click of a button are indispensable assets for clinical study sites to efficiently manage and execute essential tasks. Rapid access provisioning and effective access management to these systems are essential for ensuring timely study startup and streamlined operations.
However, as the number of systems grows for each sponsor, what initially appears as a boon can quickly become burdensome due to a multitude of challenges, leading to poor user experience, delays in study startup and operational inefficiencies.
We have often heard site professionals express concerns regarding the following issues.
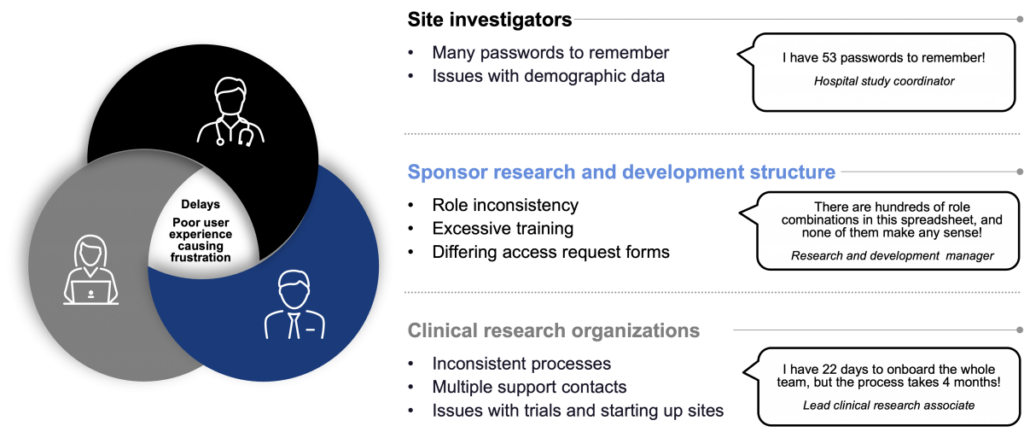
Current challenges
The current method of provisioning and managing access heavily relies on on-site staff collaborating with system owners and support teams through email and service requests—such as tickets. However, this approach highlights the challenges due to the following factors:
-
- Multiple login credentials and lack of standardized processes.
- Different support teams and varying service level agreements (SLAs) per system, each with multiple points of contact.
- Numerous hand-offs in processes resulting from multiple roles, compliance requirements and approval processes per system.
- Human errors arise from the volume, diversity and complexity of access requests, often necessitating the re-initiation of processes.
- Absence of a centralized location for accessing information and seeking assistance.
Solution: Automating access provisioning and governance
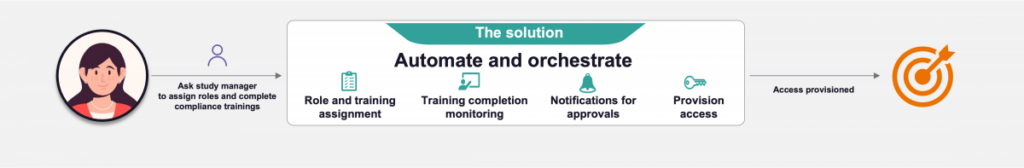
The effective management of user access often takes a back seat due to the absence of unified tools, readily available solutions and the complexity involved. Our approach addresses this challenge by implementing services, establishing an information repository and employing automation to streamline access provisioning and governance through intelligent rule-based mechanisms, thereby minimizing human intervention, process hand-offs and reducing time. This solution is founded on two primary pillars:
1. Unification of user credentials
Deploying identity management services facilitates the registration of site users into the sponsor ecosystem, thereby allowing sponsor-agnostic credentials—known as site user credentials—to seamlessly integrate with the sponsor’s single sign-on (SSO) system.

2. Automating the user access journey
The typical user access journey involves several interconnected processes that further branch out for each system.
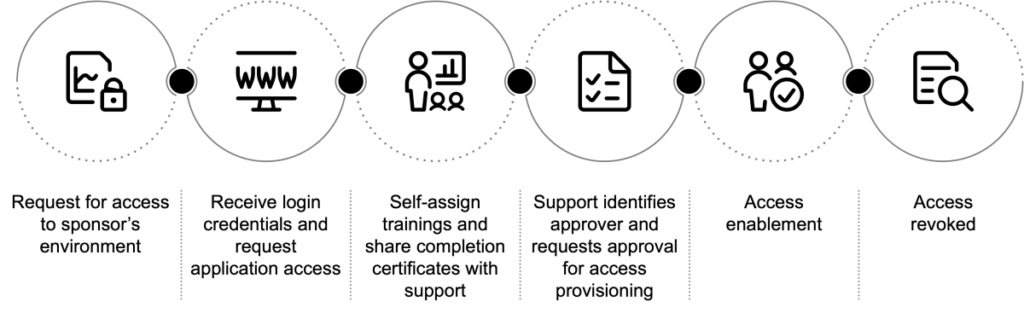
Through automation, these processes can be centralized using an orchestration engine governed by business rules. Our solution automates the following processes with minimal intervention from support teams:
-
- Invitation of users to register for unification of credentials.
- Authorization based on roles and access to clinical studies.
- Identification of required systems, their roles and compliance training.
- Assignment and monitoring of training completion.
- Email notifications to access approvers.
- Centralized hosting of important reference materials for sites, including system URLs, points of contact and best practices.
- Provisioning of access once compliance criteria are met and approvals are granted.
- Revocation of access when studies and sites are closed, or individuals leave the parent organization.
Automation reduces the time taken to provision access by up to 80% and removes the web of processes of follow-ups, incident tracking and figuring out required roles and applications.
Outcome
The two-part solution creates a unified ecosystem for streamlined and effective access provisioning and management, resulting in the following benefits:
-
- Establishment of a centralized access management system for users involved in clinical trials, covering the entire journey from invitation to revocation.
-
- Significant reduction in access provisioning time, from several months to a matter of days or weeks—up to an 80% reduction.
- Enhanced transparency to monitor users’ access journeys, identify bottlenecks and take appropriate actions.
- Decreased reliance on support staff and manual, fragmented processes.
- Improved compliance reporting through the elimination of manual audit and verification processes, replaced by a centralized, tracked audit process.
- Simplified access to information.
-
- Establishment of a centralized access management system for users involved in clinical trials, covering the entire journey from invitation to revocation.
Summary
The automated user access and identity management solution accelerates the onboarding process for site users, eliminating the difficulties associated with managing multiple credentials and routing requests through tickets and support staff. This solution offers a centralized repository of information for user data, applications, and access, facilitating efficient and automated provisioning and de-provisioning of users.
Contributors
Vinay Kumar, Director, Site Engagement, GSK
Brian Grant, Strategy Lead, GSK
Vishal Kabra, & Parijit Khokle, ZS




